Abstract
Some studies suggest that mean platelet volume (MPV) correlates with increased risk for cardiovascular morbidity and mortality. In this study, we aim to assess reproducibility, need for standardized measurements, effect of aspirin, and association with other established markers of platelet activity. Following an overnight fast, 48 healthy volunteers had weekly assessment of platelet activity and were administered aspirin 81 mg daily for 7 d between weeks 3 and 4. We investigated the influence of time between phlebotomy and MPV measurement (n=10). Reproducibility was assessed by coefficient of variation (CV) and intraclass correlation coefficient (ICC). MPV measurements were reproducible (Week 1: 10.6 fL [9.9–11], Week 2: 10.6 fL [10.0–10.9], Week 3: 10.6 fL [9.8–11]). CV was ≤4% and ICC>0.85 (p<0.001) for each comparison, indicating excellent reproducibility. There was no effect of aspirin on MPV (10.6 fL [9.8–11] versus 10.5 fL [9.9–11]; p=0.81). MPV significantly increased as time between phlebotomy and MPV measurement increased (Spearman’s rho=0.94, p=0.001). Increasing MPV tertiles was associated with collagen- and thrombin receptor-activated peptide-induced platelet aggregation but not with ADP- or arachidonic acid-induced or spontaneous platelet aggregation. In conclusion, when standardized, MPV is a reproducible marker of platelet size and not affected by low-dose aspirin. MPV is modestly associated with some, but not all, markers of platelet activity.
Keywords: Aspirin, mean platelet volume, platelet aggregation
Introduction
Platelets play a key role in atherothrombosis, and the measurement of platelet activity is independently associated with cardiovascular events and long-term mortality [1–5]. Although, light transmission aggregometry has long been considered as the historical gold standard for measurement of platelet activity, this technique requires a large volume of blood and time for processing and is associated with poor standardization and wide variability [6]. Platelets are heterogenous in size, and larger platelets are metabolically and enzymatically more active, with greater prothrombotic potential [7–9]. Mean platelet volume (MPV), a marker of platelet size, is readily available on automated hemograms, and some studies have shown significant correlations between larger MPV and increased risk for cardiovascular morbidity and mortality [10–12]. A recent study from our group found that serial measurements of MPV over time are more predictive of long-term mortality than a single measurement [13]. While increasing data has emerged demonstrating MPV as a potential biomarker in cardiovascular disease, there is no systematic data evaluating the stability of MPV over time, the effect of low-dose aspirin on MPV, and the association between MPV and other measures of platelet activity.
Methods
Study population
A group of 48 volunteers >18 years of age, who had not taken anti-platelet agents for at least 5 d, were selected to participate. These volunteers were selected after evaluation for the following exclusion criteria: (1) history of cardiovascular disease, including myocardial infarction, history of percutaneous coronary angioplasty or stent, peripheral vascular disease or stroke; (2) medications known to affect platelet function, including non-steroidal anti-inflammatory drugs, anti-histamines, and selective serotonin reuptake inhibitors, during the 5 d prior to baseline phlebotomy; (3) platelet count <100 000 or >450 000; (4) creatinine clearance <30 cc/minute; or 5) any known hemorrhagic diathesis. The New York University School of Medicine Institutional Review Board approved this study, and informed consent was obtained from each volunteer.
Study design
Baseline characteristics and medical history was obtained via direct interview, questionnaire, and physical exam. Volunteers (n=48) had assessment of platelet activity every week for four consecutive weeks and took aspirin 81 mg daily for 7 d between weeks 3 and 4. Volunteers fasted overnight and refrained from intensive exercise and tobacco use for 4 h prior to an early-morning phlebotomy to avoid any circadian changes in platelet activity.
Phlebotomy and markers of platelet activity
Blood samples were collected using a 19-gauge needle (after a 2 cc discard) into tubes containing EDTA for complete blood count (CBC) and tubes containing 3.2% (0.105 moles/L) sodium citrate for platelet activity measures. CBC, including MPV, was performed within 30 min of phlebotomy using a Sysmex XE-2100 hematology analyzer (Mundelein, Illinois, USA), which uses the impedance method to measure MPV. Ten of these volunteers had repeat MPV measurements from one blood sample at 15 n, 30, 60, 90, 120, 180, 240, 300, and 360 min after phlebotomy to assess the effect of time between phlebotomy and MPV measurement.
In all volunteers (n=48), whole blood impedance platelet aggregometry was performed within 60 min of phlebotomy using the Multiplate system (Diapharma, Franklin, Ohio). Platelet aggregation was assessed in response to saline (spontaneous platelet aggregation), 3.2 µg/mL of type 1 collagen from equine tendon, 32 µM of thrombin receptor-activated peptide (TRAP), 0.5 mM of arachidonic acid, and 6.4 µM of adenosine diphosphate (ADP). Results are expressed as percent aggregation following a 6-min evaluation.
In 47 of the volunteers, PAC-1 expression was measured by activation of the platelet integrin αIIbβ3 activity using a FITC-conjugated PAC-1 antibody (no agonist). Platelets were labeled with anti-CD42b PE antibody, and the mean fluorescent intensity (MFI) of PAC-1 expression across MPV tertiles was assessed. To further investigate the association between platelet size and platelet activity, a gated sample of 10 000 platelets from each volunteer (n=47) was separated into quartiles of platelet size, as determined by forward scatter using an Accuri C6 flow cytometer (BD Biosciences, San Jose, CA). This allowed for assessment of PAC-1 expression in each quartile of platelet size, rather than MPV.
Statistical analyses
Categorical variables are presented as proportions. Skewed continuous variables (Shapiro–Wilk) are presented as median [interquartile range] and examined using non-parametric tests. MPV was compared between weeks 1, 2, and 3 using the Kruskal–Wallis test, and reproducibility was assessed by coefficient of variation (CV) and intraclass correlation coefficient (ICC). Difference between MPV pre- (week 3) and post- (week 4) aspirin was measured with the Wilcoxon Signed Rank test. Correlation between time from phlebotomy and average MPV obtained from 10 individuals at nine different time points was assessed using Spearman’s test. Platelet aggregation and PAC-1 was compared across MPV tertiles (1st tertile n=16; 2nd tertile n=15; 3rd tertile n=17) using non-parametric independent sample tests from blood samples obtained during week 1 (Kruskal–Wallis for 3-way comparisons and Mann–Whitney for comparisons between 1st and 3rd MPV quartile). Analysis was repeated as a correlation between measures of platelet activity and MPV using Spearman’s test. Platelet size was divided into four equal distributions based on platelet size as determined by the forward scatter. Mean fluorescent intensity of PAC-1 was assessed overall and for each distribution based on platelet size. Significance was set at p<0.05.
Results
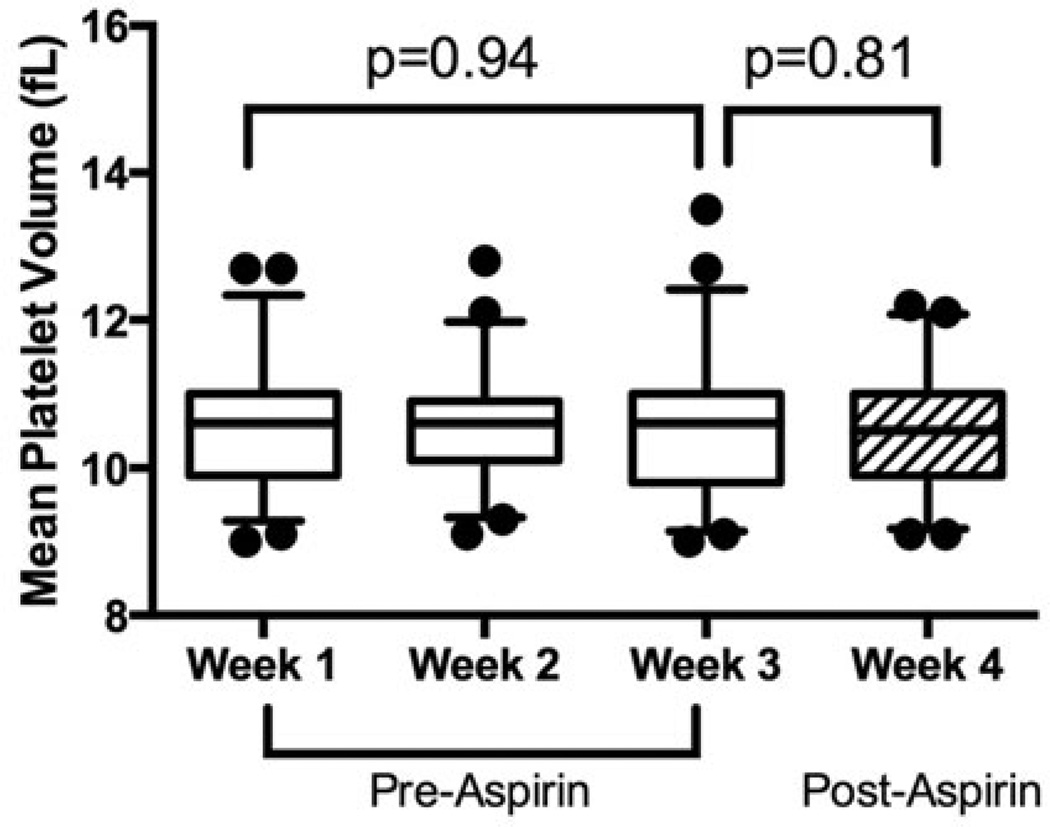
Baseline characteristics of the study population are shown in Table I. MPV values were similar between weeks 1, 2, and 3 (median [interquartile range, IQR] 10.6 fL [9.9–11.0] in week 1, 10.6 fL [10.0–10.9] in week 2, and 10.6 fL [9.8–11.0] in week 3, p=0.94; Figure 1). Reproducibility was excellent with CV ≤4% and ICC>0.85 (p<0.001) for each comparison (Week 1 and 2: CV 3.4%, ICC 0.95; Week 2 and 3: CV 3.9%, ICC 0.93; Week 1 and 3: CV 4.0%, ICC 0.92). After 7 d of aspirin therapy, there was no significant change in MPV (pre-aspirin 10.6 fL [9.8–11.0] versus. post-aspirin 10.5 fL [9.9–11.0], p=0.81; Figure 1).
Table I.
Baseline characteristics.
| Volunteers (n =48) | |
|---|---|
| Age (years) | 27 [23–32] |
| Male (%) | 50 |
| Race (%) | |
| White | 56.3 |
| Black | 2.1 |
| Asian | 31.3 |
| Ethnicity (%) | |
| Hispanic | 6.5 |
| Body mass index (kg/m2) | 24 [22–26] |
| Tobacco use (%) | 25.5 |
| Current | 6.3 |
| Family history of cardiovascular disease (%) | 14.6 |
Figure 1.
Reproducibility and effect of aspirin on MPV. Reproducibility of MPV was assessed in healthy volunteers (n=48) by weekly measures for 3 weeks under standardized conditions. Asprin 81 mg was then administered for 7 d between week 3 and week 4 and MPV was re-assessed. Data presented as median [interquartile range] (box) and range (whiskers); compared between Weeks 1, 2, and 3 using the Kruskal–Wallis test; and compared between Weeks 3 and 4 using the Wilcoxon Signed Rank test.
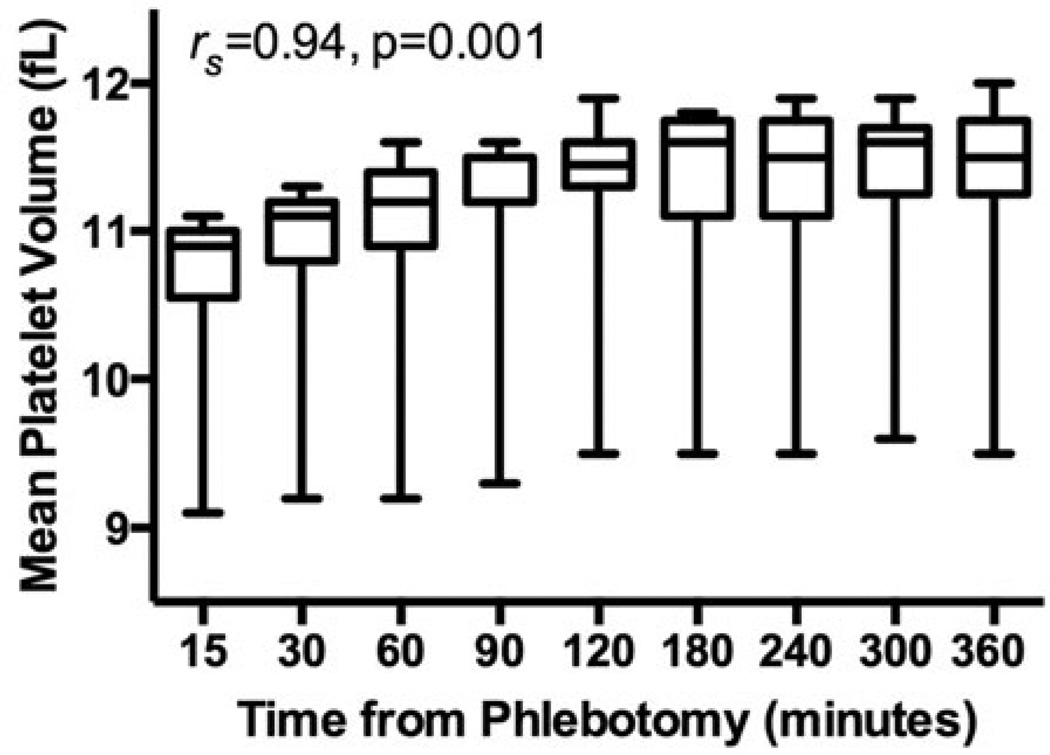
To assess the pre-analytical effect of time between phlebotomy and MPV measurement, MPV was measured at different post-phlebotomy time-points in a subset of 10 volunteers (15 min: 10.9 fL [10.5–11.0], 30 min: 11.1 fL [10.8–11.2], 60 min: 11.2 fL [10.9–11.4], 90 min: 11.2 fL [11.2–11.5], 120 min: 11.4 fL [11.3– 11.6], 180 min: 11.6 fL [11.1–11.7], 240 min: 11.5 fL [11.1–11.7], 300 min: 11.6 fL [11.2–11.7], 360 min: 11.5 fL [11.2–11.7]). MPV significantly increased as time between phlebotomy and MPV measurement increased (Spearman’s rho of averaged data 0.941, p=0.001) (Figure 2).
Figure 2.
Effect of time between phlebotomy and MPV measurements. MPV was assessed in healthy volunteers (n=10) at nine different time points from the time of phlebotomy. Correlation is shown between time from phlebotomy and average MPV levels using Spearman’s test. Data presented as median [interquartile range] (box) and range (whiskers).
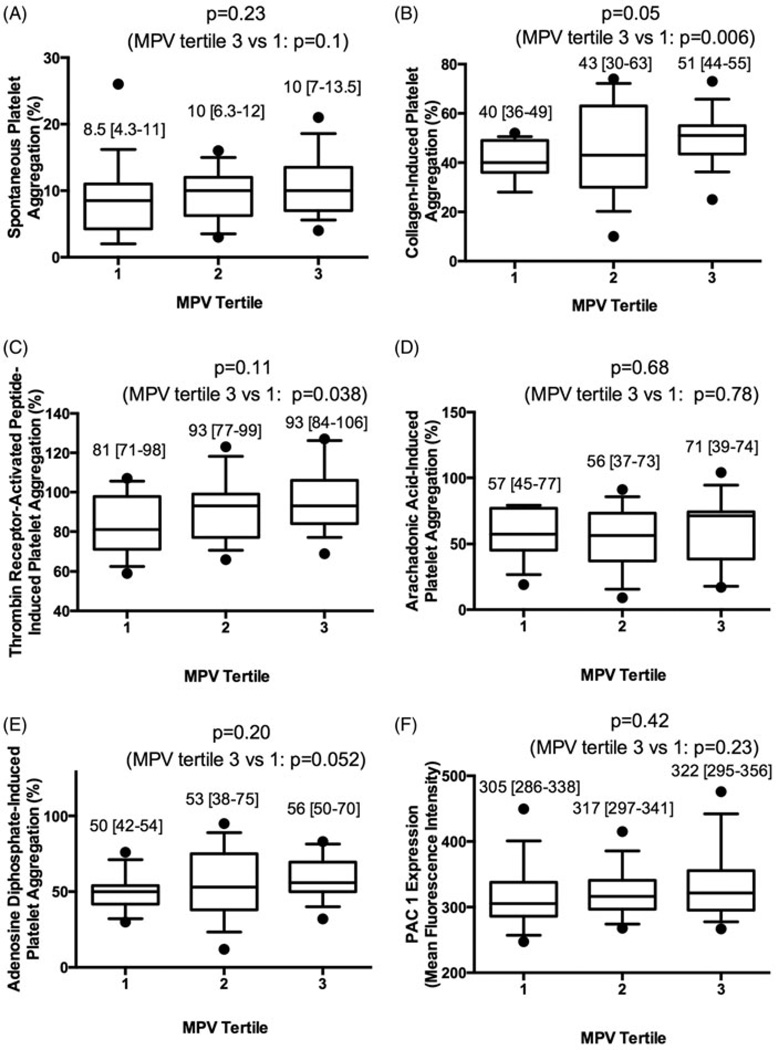
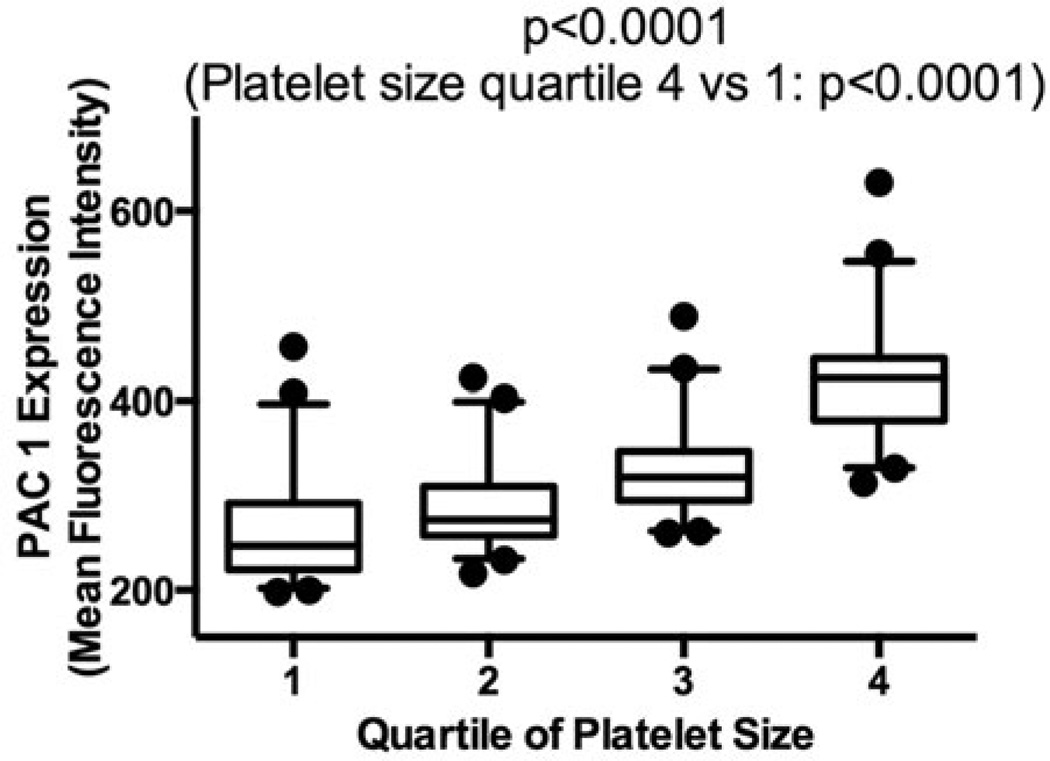
Platelet activity, as measured by platelet aggregation and PAC-1 expression, across MPV tertiles are shown in Figure 3. While markers were numerically higher in subjects with increased MPV, only collagen-induced and TRAP-induced platelet aggregation was significantly higher in participants in the 3rd compared with the 1st MPV tertile. When analyzed as continuous variable correlations, only collagen-induced (Spearman’s rho 0.33, p=0.02) and spontaneous (Spearman’s rho 0.32, p=0.03) platelet aggregation significantly correlated with MPV; TRAP- (Spearman’s rho 0.21, p=0.16), Arachidonic Acid- (Spearman’s rho 0.06, p=0.68), ADP-induced platelet aggregation (Spearman’s rho 0.23, p=0.13), and PAC-1 expression (Spearman’s rho 0.18, p=0.23) was not statistically significant. We further investigated platelet activation across quartiles of platelet size within each individual, and PAC-1 expression was significantly increased in larger platelets (1st quartile 247 [222–292], 2nd quartile 274 [258–310], 3rd quartile 319 [295–347], 4th quartile 423 [380–445], p<0.001; Figure 4).
Figure 3.
Platelet aggregation and PAC-1 expression across tertiles of MPV. Platelet aggregation was assessed in response to (A) saline, (B) 3.2 µg/mL of collagen, (C) 32 µM of thrombin receptor-activated peptide, (D) 0.5 mM of arachidonic acid, and (E) 6.4 µM of adenosine diphosphate following a 6-min evaluation across tertiles of MPV in healthy volunteers (n=48). (F) PAC-1 expression (mean fluorescence intensity) was assessed in response to saline across tertiles of MPV in healthy volunteers (n=47). PAC-1 expression was also assessed in response to epinephrine (546 [399–668], 557 [416–727], 604 [475–715]; p=0.51, p=0.27 between 1st and 3rd MPV tertile) and adenosine diphosphate (630 [458–844], 739 [502–1046], 799 [579–1132]; p=0.43, p=0.25 between 1st and 3rd MPV tertile) (not shown in figure). Data presented as median [interquartile range] (box) and range (whiskers); compared across tertiles using the Kruskal–Wallis test; and compared between 1st and 3rd tertile of platelet size using the Mann–Whitney test.
Figure 4.
PAC-1 expression across quartiles of platelet size from a gated sample of 10 000 platelets. A gated sample of 10 000 platelets from each healthy volunteer (n=47) was separated by quartiles of platelet size based on forward scatter, and PAC-1 binding (mean fluorescence intensity) was assessed in response to saline in each quartile. Data presented as median [interquartile range] (box) and range (whiskers); compared across quartiles using the Kruskal–Wallis test; and compared between 1st and 4th quartile of platelet size using the Mann–Whitney test.
Discussion
This study demonstrates that, when standardized, MPV measurements are reproducible and not affected by the use of low-dose aspirin. Prior to this study, the potential confounding effects of anti-platelet agents on MPV measurements were unclear. One study showing patients with an elevated MPV and aspirin resistance (assessed using PFA-100) had a significantly increased rate of the composite endpoint of death, myocardial infarction, and target vessel revascularization [14]. Our results in a healthy population are consistent with the only other published study retrospectively demonstrating no effect of low-dose aspirin therapy on MPV in patients with paroxysmal atrial fibrillation [15]. While our prospective analysis is not adequately powered to detail the effects of aspirin therapy on MPV measurements, we demonstrate not even a hint of effect. A recent single-center prospective analysis by de Luca et al. demonstrated an increase in MPV after starting dual anti-platelet therapy in patients with acute coronary syndrome [16]. However, they did not tease out the individual effects of clopidogrel versus aspirin.
When evaluating platelet activity, several pre-analytical considerations should be made, including the method of venipuncture, choice of anti-coagulant, and time interval between phlebotomy and measurement, but there is little data to support a detailed standardized protocol of measurement [17, 18]. In this study, all pre-analytical components were standardized. We used an automated hematology analyzer that uses the impedance method to measure MPV, which determines particle size based on the conduction properties of cells. The other commonly used automated technique of platelet counting and MPV measurement, the light scatter flow cytometry method, uses light scatter produced by a laser illuminating the cells, which is then measured at two angles and converted to electrical pulses. The number of pulses and amplitude correspond to cell count and volume, respectively. MPV has been shown to be up to 40% different when measured using the light scatter versus impedance methods, making the type of hematology analyzer used an important preanalytical consideration when measuring MPV [19]. We also studied in detail the effect of time between phlebotomy and MPV measurement in a subset of patients. Our data is consistent with other studies demonstrating the time-dependence of MPV measurement due to swelling of platelets in EDTA until 120 min [20, 21].
Lastly, this is the only study to systematically evaluate the relationship between MPV and a variety of measures of platelet activity, and takes a closer look at individual platelet size and platelet activity. We demonstrated that MPV is associated with some, but not all, metrics of platelet activity evaluated in this study. These results are consistent with de Luca’s analysis demonstrating no relationship between baseline MPV and platelet activity measured by impedance aggregometry using the agonist arachidonic acid [16]. It is possible that this study is underpowered to make any conclusions whether MPV is more strongly associated with platelet aggregation to one agonist versus another. Future adequately powered studies are needed to assess whether a true difference between MPV and different platelet agonists exist. However, while multiple studies have demonstrated increased MPV to be a predictor of adverse clinical outcomes in large populations [7–9], we suggest that this may not be as useful in an individual patient.
Limitations
This study is not designed to address long-term follow-up or associations with clinical endpoints but does provide insight into future study designs utilizing MPV as the biomarker of interest. Second, we selected whole blood impedance platelet aggregometry and PAC-1 expression via flow cytometry as comparative measures of platelet activity, but there are other methods of measuring platelet activity that were not chosen for this study. Similarly, we only used one concentration of agonist to evaluate maximal extent of platelet aggregation, and this may not be sensitive enough to evaluate hyper-reactivity. Third, the effect of a longer duration of aspirin or other more potent anti-platelet medications such as clopidogrel, prasugrel, and ticagrelor were not studied. Fourth, although tobacco use is a known influence on platelet activity and tobacco users were not excluded from participation, only a minority (6%) of subjects in this study were current tobacco users. Nonetheless, this is the only study, to date, to systematically evaluate the reproducibility of standardized MPV measurements, the effect of aspirin on MPV measurements, the association of MPV with different markers of platelet activity, and the association of individual platelet size with platelet activity (measured by PAC-1 binding on flow cytometry).
Conclusions
When standardized, MPV is a reproducible marker of platelet size. MPV is not affected by low-dose aspirin but is significantly affected by time between phlebotomy and measurement, emphasizing the need for standardization of MPV measurements. Platelet size, rather than MPV, may be a more useful marker of platelet activity in an individual patient. MPV measurements should be standardized and larger studies correlating standardized MPV measurements with clinical outcomes are needed.
Acknowledgments
Dr Shah was partially funded by an NIH/NHLBI grant (T32HL098129). Dr Berger was partially funded by an American Heart Association Fellow to Faculty Award (0775074N) and a Doris Duke Clinical Scientist Award (2010055).
Footnotes
Declaration of interest
The authors have no conflicts of interest to report.
References
- 1.Fuster V, Badimon L, Badimon JJ, Chesebro JH. The pathogenesis of coronary artery disease and the acute coronary syndromes (2) N Engl J Med. 1992;326:310–318. doi: 10.1056/NEJM199201303260506. [DOI] [PubMed] [Google Scholar]
- 2.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 3.Michelson AD. Platelet function testing in cardiovascular diseases. Circulation. 2004;110:e489–e493. doi: 10.1161/01.CIR.0000147228.29325.F9. [DOI] [PubMed] [Google Scholar]
- 4.Gurbel PA, Becker RC, Mann KG, Steinhubl SR, Michelson AD. Platelet function monitoring in patients with coronary artery disease. J Am Coll Cardiol. 2007;50:1822–1834. doi: 10.1016/j.jacc.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 5.Trip MD, Cats VM, van Capelle FJ, Vreeken J. Platelet hyperreactivity and prognosis in survivors of myocardial infarction. N Engl J Med. 1990;322:1549–1554. doi: 10.1056/NEJM199005313222201. [DOI] [PubMed] [Google Scholar]
- 6.Cattaneo M, Hayward CP, Moffat KA, Pugliano MT, Liu Y, Michelson AD. Results of a worldwide survey on the assessment of platelet function by light transmission aggregometry: A report from the platelet physiology subcommittee of the SSC of the ISTH. J Thromb Haemost. 2009;7:1029. doi: 10.1111/j.1538-7836.2009.03458.x. [DOI] [PubMed] [Google Scholar]
- 7.Chu SG, Becker RC, Berger PB, Bhatt DL, Eikelboom JW, Konkle B, Mohler ER, Reilly MP, Berger JS. Mean platelet volume as a predictor of cardiovascular risk: A systematic review and meta-analysis. J Thromb Haemost. 2010;8:148–156. doi: 10.1111/j.1538-7836.2009.03584.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huczek Z, Kochman J, Filipiak KJ, Horszczaruk GJ, Grabowski M, Piatkowski R, Wilczynska J, Zielinski A, Meier B, Opolski G. Mean platelet volume on admission predicts impaired reperfusion and long-term mortality in acute myocardial infarction treated with primary percutaneous coronary intervention. J Am Coll Cardiol. 2005;46:284–290. doi: 10.1016/j.jacc.2005.03.065. [DOI] [PubMed] [Google Scholar]
- 9.Goncalves SC, Labinaz M, Le May M, Glover C, Froeschl M, Marquis JF, O’Brien E, Shukla D, Ruchin P, Sookur D, et al. Usefulness of mean platelet volume as a biomarker for long-term outcomes after percutaneous coronary intervention. Am J Cardiol. 2011;107:204–209. doi: 10.1016/j.amjcard.2010.08.068. [DOI] [PubMed] [Google Scholar]
- 10.Karpatkin S. Heterogeneity of human platelets. II. Functional evidence suggestive of young and old platelets. J Clin Invest. 1969;48:1083–1087. doi: 10.1172/JCI106064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Karpatkin S. Heterogeneity of human platelets. I. Metabolic and kinetic evidence suggestive of young and old platelets. J Clin Invest. 1969;48:1073–1082. doi: 10.1172/JCI106063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kamath S, Blann AD, Lip GY. Platelet activation: Assessment and quantification. Eur Heart J. 2001;22:1561–1571. doi: 10.1053/euhj.2000.2515. [DOI] [PubMed] [Google Scholar]
- 13.Shah B, Oberweis B, Tummala L, Amoroso N, Lobach I, Gross E, Sedlis SP, Berger JS. Mean platelet volume and long-term mortality in patients undergoing percutaneous coronary intervention. Am J Cardiol. 2103;111:185–189. doi: 10.1016/j.amjcard.2012.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aksu H, Ozer O, Unal H, Hobikoglu G, Norgaz T, Buturak A, Soylu O, Narin A. Significance of mean platelet volume on prognosis of patients with and without aspirin resistance in settings of non-ST-segment elevated acute coronary syndromes. Blood Coagul Fibrinolysis. 2009;20:686–693. doi: 10.1097/MBC.0b013e32833161ac. [DOI] [PubMed] [Google Scholar]
- 15.Colkesen Y, Coskun I, Muderrisoglu H. The effect of aspirin on mean platelet volume in patients with paroxysmal atrial fibrillation. Platelets. 2013;24:263–266. doi: 10.3109/09537104.2012.682106. [DOI] [PubMed] [Google Scholar]
- 16.De Luca G, Secco GG, Iorio S, Verdoia M, Bellomo G, Marino P. Short-term effects of aspirin and clopidogrel on mean platelet volume among patients with acute coronary syndromes. A single-center prospective study. Blood Coagul Fibrinolysis. 2012;23:756–759. doi: 10.1097/MBC.0b013e328358e941. [DOI] [PubMed] [Google Scholar]
- 17.Lima-Oliveira G, Lippi G, Salvagno GL, Montagnana M, Poli G, Solero GP, Picheth G, Guidi GC. K(3)EDTA vacuum tubes validation for routine hematological testing. ISRN Hematol. 2012;2012:875357. doi: 10.5402/2012/875357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latger-Cannard V, Hoarau M, Salignac S, Baumgart D, Nurden P, Lecompte T. Mean platelet volume: Comparison of three analysers towards standardization of platelet morphological phenotype. Int J Lab Hematol. 2012;34:300–310. doi: 10.1111/j.1751-553X.2011.01396.x. [DOI] [PubMed] [Google Scholar]
- 19.Trowbridge EA, Reardon DM, Hutchinson D, Pickering C. The routine measurement of platelet volume: A comparison of light-scattering and aperture-impedance technologies. Clin Phys Physiol Meas. 1985;6:221–238. doi: 10.1088/0143-0815/6/3/003. [DOI] [PubMed] [Google Scholar]
- 20.Bath PM. The routine measurement of platelet size using sodium citrate alone as the anticoagulant. Thromb Haemost. 1993;70:687–690. [PubMed] [Google Scholar]
- 21.Lance MD, van Oerle R, Henskens YM, Marcus MA. Do we need adjusted mean platelet volume measurements? Lab Hematol. 2010;16:28–31. doi: 10.1532/LH96.10011. [DOI] [PubMed] [Google Scholar]