Cancer vaccine strategies require the transfer of tumor-associated antigens from tumor cells to antigen-presenting cells (APCs) for degradation and presentation of peptide fragments on major histocompatibility complex class I molecules. Dendritic cells (DCs) are the most potent APCs for priming T cells, and they can activate B cells to initiate antibody responses.1 However, DCs have several limitations related to their production, in that they are highly differentiated cells with a limited replicative potential and a short life span. In addition to the complexities associated with manufacturing therapeutic numbers of DCs, vaccine efficacy in clinical trials has generally fallen short of expectations, according to results from preclinical animal models.
In principle, genetic engineering can overcome some of the limitations involved with the use of natural DCs. DCs are most commonly prepared by adding granulocyte-macrophage colony-stimulating factor (GM-CSF) and interleukin-4 (IL-4) to cultures of DC precursor cells. GM-CSF promotes the differentiation of DCs from mouse bone marrow cultures, whereas IL-4 downregulates CD14 and prevents macrophage overgrowth. DCs are amenable to gene modification by multiple viral and nonviral vector approaches. Genetically modified mouse DCs that secrete IL-4 display increased surface expression of major histocompatibility complex class II and costimulatory molecules, and also secrete more bioactive IL-12p70 (ref. 2). Lentiviral vectors can efficiently introduce antigens into DCs,3 and lentiviral vector– mediated transduction may be a desirable approach because it does not have significant effects on DC maturation or function. For example, in a side-by-side comparison, DCs modified by electroporation were impaired as compared with their lentivirally transduced and unmodified counterparts, as judged by IL-12p70 production.4
Stripecke and her team of investigators (Koya et al.5) have carried out a series of studies to extend the promise of genetically engineered DCs. They previously observed that lentiviral-mediated expression of GM-CSF and IL-4 was sufficient to convert cultures of human monocytes to DC-like cells as judged by phenotype, morphology, and microscopy. Previous work by others showed that expression of GM-CSF by transfection of mouse bone marrow– derived DCs leads to enhanced antigen presentation as compared with mock-transfected cells.6 In addition to independence from exogenous cytokines, the transfected DCs unexpectedly had a prolonged in vitro life span. From a practical perspective, the gene modification shortened the required duration of cell culture. In their new study, described in this issue, Koya et al. extend previous work by transducing mouse bone marrow cells with bicistronic lentiviral vectors to introduce the cytokines GM-CSF and IL-4, as well as tumor antigens in some experiments. They show that bone marrow–derived cells can be reprogrammed to function as DCs.7 Autonomous trans-differentiation of murine bone marrow cells into functionally active APCs is demonstrated, and the authors observed that the efficiency of trafficking of the engineered DCs was generally superior to that of natural DCs grown in the presence of exogenous cytokines. It is intriguing to note that bioluminescence imaging demonstrated that the designer DCs had a 10-fold or more improved engraftment in vivo in mice, and they persisted in vivo for at least 40 days. The prolonged life span of the engineered DCs in vivo is consistent with the group’s previous in vitro studies.5 The mechanism underlying the enhanced life span is not known, and it is currently unclear whether the cells that persist long term are in fact bona fide DCs or APCs.
One surprising aspect of this new study is that the cytokine-secreting DCs proved superior to natural DCs in several assays of function. The enhanced longevity of the engineered DCs may account for this. In addition, these engineered DCs probably exert paracrine effects on bystander host APCs; these paracrine effects might be substantial, as judged by the striking lymphadenopathy observed in mice that received designer DCs.
The rationale for the inclusion of IL-4 in the cytokine mix was to prevent macrophage differentiation and promote DCs in the presence of GM-CSF. However, IL-4 might have other paracrine effects that could mediate a beneficial antitumor effect. For example, Hurford and colleagues have shown that retroviral-mediated expression of IL-4 in tumor deposits can elicit potent antitumor effects.8 Thus, there may be promise for the intratumoral injection of reprogrammed DCs or the lentiviral vectors themselves that express IL-4 and GM-CSF, in that they seem to mediate both autocrine and paracrine effects. It will be important to learn to what extent the engineered DCs promote T-helper type 1-like effects that promote antitumor immunity and whether they promote the development of regulatory T cells, because natural DCs can efficiently promote regulatory T-cell activation and expansion.9
This work raises several questions that must be addressed before this approach can be translated into clinical testing. First, significant safety issues arise regarding the use of lentiviral vectors, including the potential for insertional oncogenesis as well as the consequences of constitutive cytokine expression. With regard to the former issue, it is reassuring that the initial studies using lentiviral vectors in humans have uncovered no safety issues thus far.10 With regard to the latter issue, it will be important to regulate cytokine expression. For example, transgenic mice that express constitutive GM-CSF develop a fatal wasting syndrome.11 Retroviral vector-mediated gene delivery of GM-CSF into factor-dependent myeloid cells converted them to factor independence, but this was not associated with leukemia.12 It is reassuring that in the present study the mice engrafted with programmed DCs have shown no evidence of hematologic malignancy.7 Furthermore, it is possible that incorporation of a conditional suicide construct into the engineering schema would allay any safety concerns that may arise.
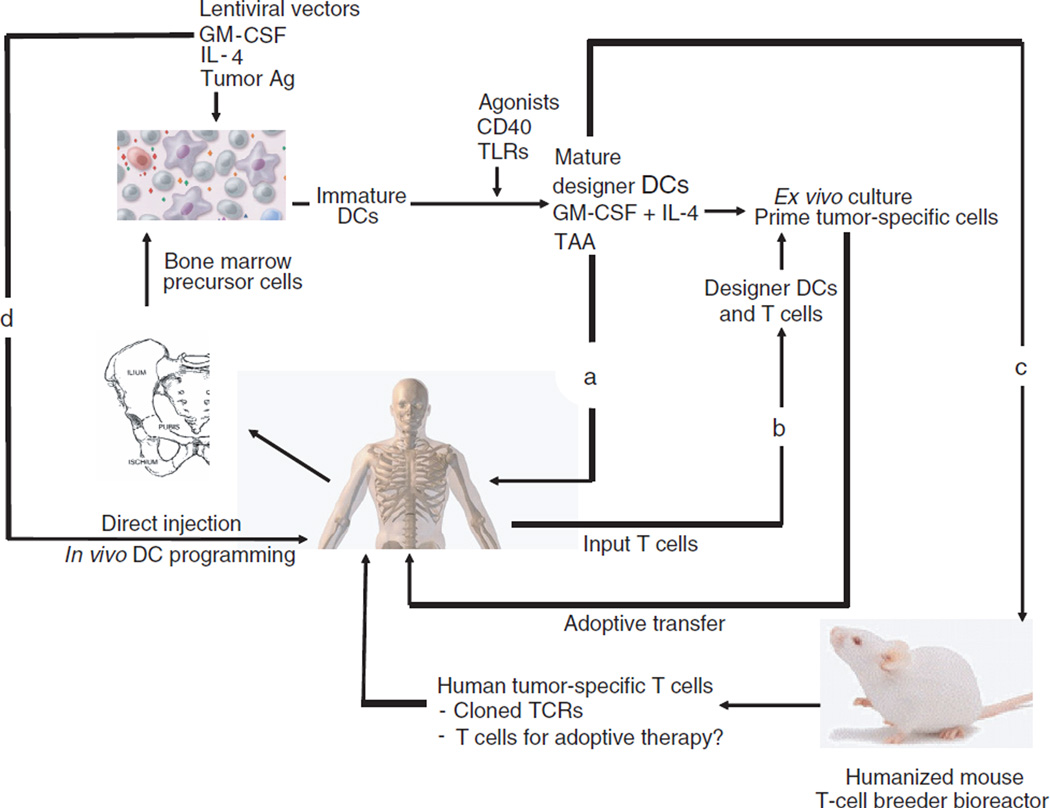
The approach pioneered by Koya and colleagues has multiple potential applications (Figure 1). As a direct extension of the present studies in mice, human bone marrow–derived or peripheral blood monocyte–like precursor cells could be engineered to constitutively express cytokines as well as tumor antigens. Subsequent to maturation with an agent such as CD40L, the cells could then serve as a therapeutic tumor vaccine with the potential for enhanced longevity and improved trafficking as compared with conventional DCs (Figure 1a). It is interesting to note that experiments of nature teach that long-lived DCs in humans seem to have an enhanced potential to cause autoimmune syndromes. Patients with congenital caspase 10 deficiency have impaired apoptosis and long-lived DCs in vitro and, clinically, a predisposition toward autoimmune syndromes. 13 Because one of the holy grails in tumor immunology has been the quest to overcome self-tolerance and tumor tolerance, there is the potential that pharmacologically enhanced DCs such as those developed by the Stripecke team could lead to progress in this area. In addition, the engineered APCs could be injected by several routes, including intratumoral administration, which has been underexplored by the field and yet has promise in preclinical studies with natural DCs.14
Figure 1. Possible applications of programmed DCs.
A bone marrow sample obtained from a normal donor or a tumor-bearing patient is placed in culture, and DC precursors are transduced with a cocktail of lentiviral vectors to express cytokines GM-CSF and IL-4 as well as tumor antigen(s) (TAA). (a) In the approach used by Koya et al.,7 a clinical application would be to use the transduced cell population for prophylactic or therapeutic tumor vaccine by injection of engineered DCs by various routes. (b) An ex vivo application would be to use the engineered DCs to prime tumor-reactive T cells for later adoptive transfer therapy. In this scenario it is likely that maturation and activation (e.g., CD40L stimulation) of the DCs would enhance the efficiency. (c) A humanized mouse approach could be used to generate populations of tumor-reactive T cells as a source of tumor-specific T cells or, conceivably, to generate T cells for therapeutic use by engrafting mice with CD34 cells or cord blood T cells, and using the engineered DCs to induce the outgrowth of large numbers of tumor-reactive human T cells. TCRs, T-cell receptors. (d) Direct administration of lentiviral vectors may result in in situ programming of DC precursor cells in vivo. Ag, antigen.
Ex vivo applications of the engineered DCs can also be envisioned (Figure 1b). Engineered DCs could be produced and incorporated into culture systems to prime and expand T cells for adoptive transfer therapy; it is expected that this approach would be technically facilitated by the rapid production of the DCs, and it is likely that the programmed DCs would be more efficient at priming and expanding tumor-reactive T cells.
A more sophisticated strategy could conceivably permit engineered DCs to be used to generate high-avidity antitumor T cells (Figure 1c). Transfer of human hematopoietic stem cells into immune-deficient mice yields animals engraft ed with robust levels of human T cells and APCs.15 Because these T cells are not edited by negative selection in the human thymus, they could contain T cells with strong reactivity against tumor epitopes, cells that are normally eliminated during thymic maturation as a result of self-reactivity. In this scenario xenografted mice could be vaccinated with programmed DCs and then used as bioreactors to produce high-avidity, highly potent antitumor T cells. Finally, it is possible that lentiviral vector technology can be improved sufficiently that programmed DCs could be directly converted or differentiated in vivo by targeted transduction of resident DC precursors (Figure 1d). For example, previous studies in mice using a lentiviral vector to express NY-ESO-1 have shown that direct lentiviral vector injection was similar in potency to the injection of in vitro– transduced DCs.16
The studies of Koya et al. point out a new path to take in the development of therapeutic cancer vaccines. Additional studies are warranted on the safety of the approach as noted earlier. Other studies should take into account the differences between mouse and human DC biology. Relevant here may be the recent finding that distinct subsets of DCs are dedicated to antigen processing and presentation for CD4+ and CD8+ T cells.17 Finally, in addition to improving efficacy, a major barrier to the development of cancer vaccines has been related to the economics of delivering personalized medicine. The next generation of designer DCs are attractive in that they have the potential to provide shortcuts to the ex vivo manufacturing procedure, so that it may be possible to simplify the complex process currently required for production of natural DCs. Further progress in designer DCs has the potential to advance the feasibility of developing effective vaccines for several cancers and chronic infections.
This is a commentary on article Koya RC, Kimura T, Ribas A, Rozengurt N, Lawson GW, Faure-Kumar E, Wang HJ, Herschman H, Kasahara N, Stripecke R. Lentiviral vector-mediated autonomous differentiation of mouse bone marrow cells into immunologically potent dendritic cell vaccines. Mol Ther. 2007;15(5):971-80.
REFERENCES
- 1.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–252. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 2.Kaneko K, Wang Z, Kim SH, Morelli AE, Robbins PD, Thomson AW. Dendritic cells genetically engineered to express IL-4 exhibit enhanced IL-12p70 production in response to CD40 ligation and accelerate organ allograft rejection. Gene Ther. 2003;10:143–152. doi: 10.1038/sj.gt.3301872. [DOI] [PubMed] [Google Scholar]
- 3.Schroers R, Sinha I, Segall H, Schmidt-Wolf IG, Rooney CM, Brenner MK, et al. Transduction of human PBMC-derived dendritic cells and macrophages by an HIV-1–based lentiviral vector system. Mol Ther. 2000;1:171–179. doi: 10.1006/mthe.2000.0027. [DOI] [PubMed] [Google Scholar]
- 4.Dullaers M, Breckpot K, Van Meirvenne S, Bonehill A, Tuyaerts S, Michiels A, et al. Side-by-side comparison of lentivirally transduced and mRNA-electroporated dendritic cells: implications for cancer immunotherapy protocols. Mol Ther. 2004;10:768–779. doi: 10.1016/j.ymthe.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 5.Koya RC, Weber JS, Kasahara N, Lau R, Villacres MC, Levine AM, et al. Making dendritic cells from the inside out: lentiviral vector-mediated gene delivery of granulocyte-macrophage colony-stimulating factor and interleukin 4 into CD14+ monocytes generates dendritic cells in vitro. Hum Gene Ther. 2004;15:733–748. doi: 10.1089/1043034041648381. [DOI] [PubMed] [Google Scholar]
- 6.Curiel-Lewandrowski C, Mahnke K, Labeur M, Roters B, Schmidt W, Granstein RD, et al. Transfection of immature murine bone marrow–derived dendritic cells with the granulocyte-macrophage colony-stimulating factor gene potently enhances their in vivo antigen-presenting capacity. J Immunol. 1999;163:174–183. [PubMed] [Google Scholar]
- 7.Koya RC, Kimura T, Ribas A, Rozengurt N, Lawson GW, Faure-Kumar E, et al. Lentiviral vector-mediated autonomous differentiation of mouse bone marrow cells into immunologically potent dendritic cell vaccines. Mol Ther. 2007;15:971–980. doi: 10.1038/mt.sj.6300126. [DOI] [PubMed] [Google Scholar]
- 8.Hurford RK, Jr, Dranoff G, Mulligan RC, Tepper RI. Gene therapy of metastatic cancer by in vivo retroviral gene targeting. Nat Genet. 1995;10:430–435. doi: 10.1038/ng0895-430. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee DK, Dhodapkar MV, Matayeva E, Steinman RM, Dhodapkar KM. Expansion of FOXP3 high regulatory T cells by human dendritic cells (DCs) in vitro and after injection of cytokinematured DCs in myeloma patients. Blood. 2006;108:2655–2661. doi: 10.1182/blood-2006-03-011353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Levine BL, Humeau LM, Boyer J, MacGregor RR, Rebello T, Lu X, et al. Gene transfer in humans using a conditionally replicating lentiviral vector. Proc Natl Acad Sci USA. 2006;103:17372–17377. doi: 10.1073/pnas.0608138103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lang RA, Metcalf D, Cuthbertson RA, Lyons I, Stanley E, Kelso A, et al. Transgenic mice expressing a hemopoietic growth factor gene (GM-CSF) develop accumulations of macrophages, blindness, and a fatal syndrome of tissue damage. Cell. 1987;51:675–686. doi: 10.1016/0092-8674(87)90136-x. [DOI] [PubMed] [Google Scholar]
- 12.Laker C, Stocking C, Bergholz U, Hess N, De Lamarter JF, Ostertag W. Autocrine stimulation after transfer of the granulocyte/macrophage colony-stimulating factor gene and autonomous growth are distinct but interdependent steps in the oncogenic pathway. Proc Natl Acad Sci USA. 1987;84:8458–8462. doi: 10.1073/pnas.84.23.8458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Zheng L, Lobito A, Chan FK, Dale J, Sneller M, et al. Inherited human caspase-10 mutations underlie defective lymphocyte and dendritic cell apoptosis in autoimmune lymphoproliferative syndrome type II. Cell. 1999;98:47–58. doi: 10.1016/S0092-8674(00)80605-4. [DOI] [PubMed] [Google Scholar]
- 14.Candido KA, Shimizu K, McLaughlin JC, Kunkel R, Fuller JA, Redman BG, et al. Local administration of dendritic cells inhibits established breast tumor growth: implications for apoptosis-inducing agents. Cancer Res. 2001;61:228–236. [PubMed] [Google Scholar]
- 15.Shultz LD, Lyons BL, Burzenski LM, Gott B, Chen X, Chaleff S, et al. Human lymphoid and myeloid cell development in NOD/LtSz-scid IL2R gamma null mice engrafted with mobilized human hemopoietic stem cells. J Immunol. 2005;174:6477–6489. doi: 10.4049/jimmunol.174.10.6477. [DOI] [PubMed] [Google Scholar]
- 16.Palmowski MJ, Lopes L, Ikeda Y, Salio M, Cerundolo V, Collins MK. Intravenous injection of a lentiviral vector encoding NY-ESO-1 induces an effective CTL response. J Immunol. 2004;172:1582–1587. doi: 10.4049/jimmunol.172.3.1582. [DOI] [PubMed] [Google Scholar]
- 17.Dudziak D, Kamphorst AO, Heidkamp GF, Buchholz VR, Trumpfheller C, Yamazaki S, et al. Differential antigen processing by dendritic cell subsets in vivo. Science. 2007;315:107–111. doi: 10.1126/science.1136080. [DOI] [PubMed] [Google Scholar]