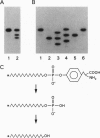
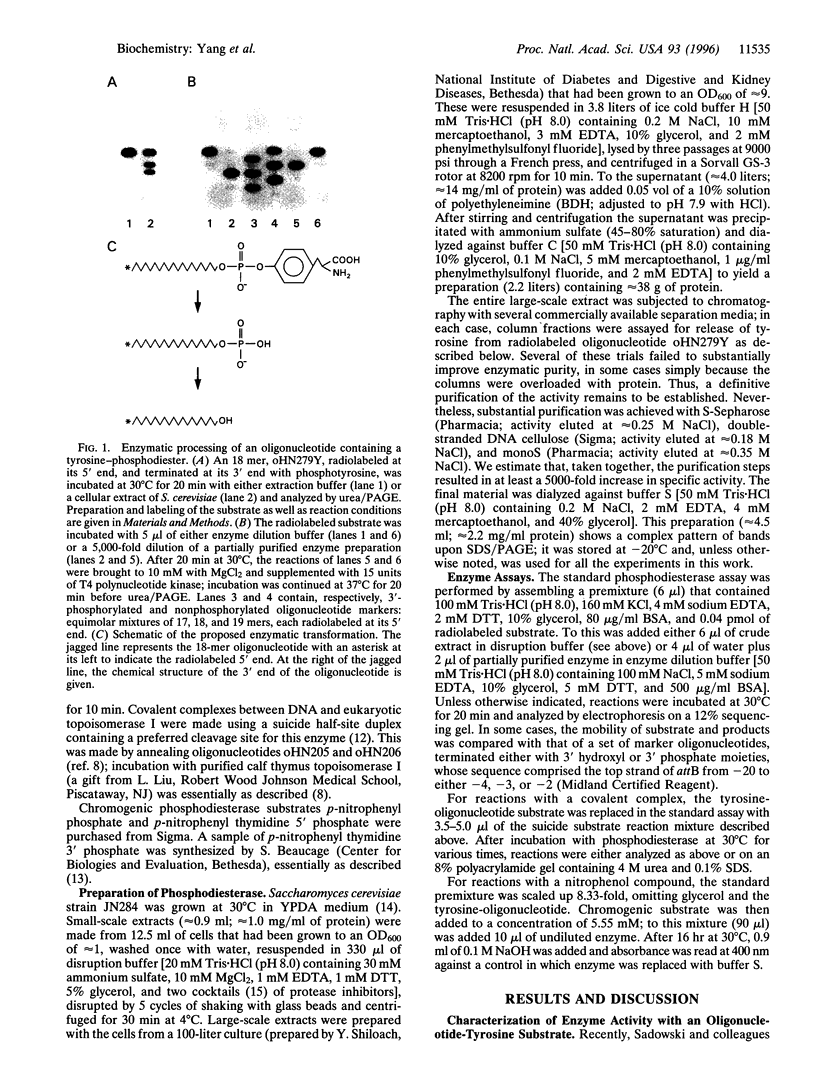
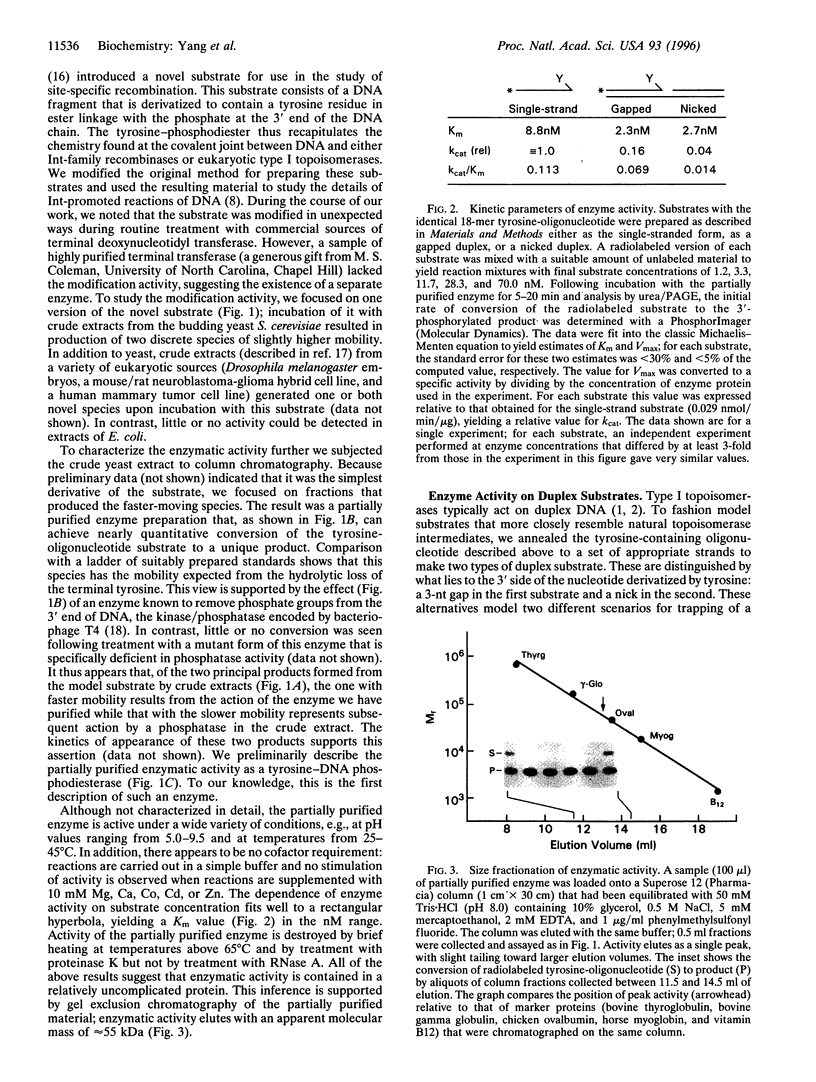
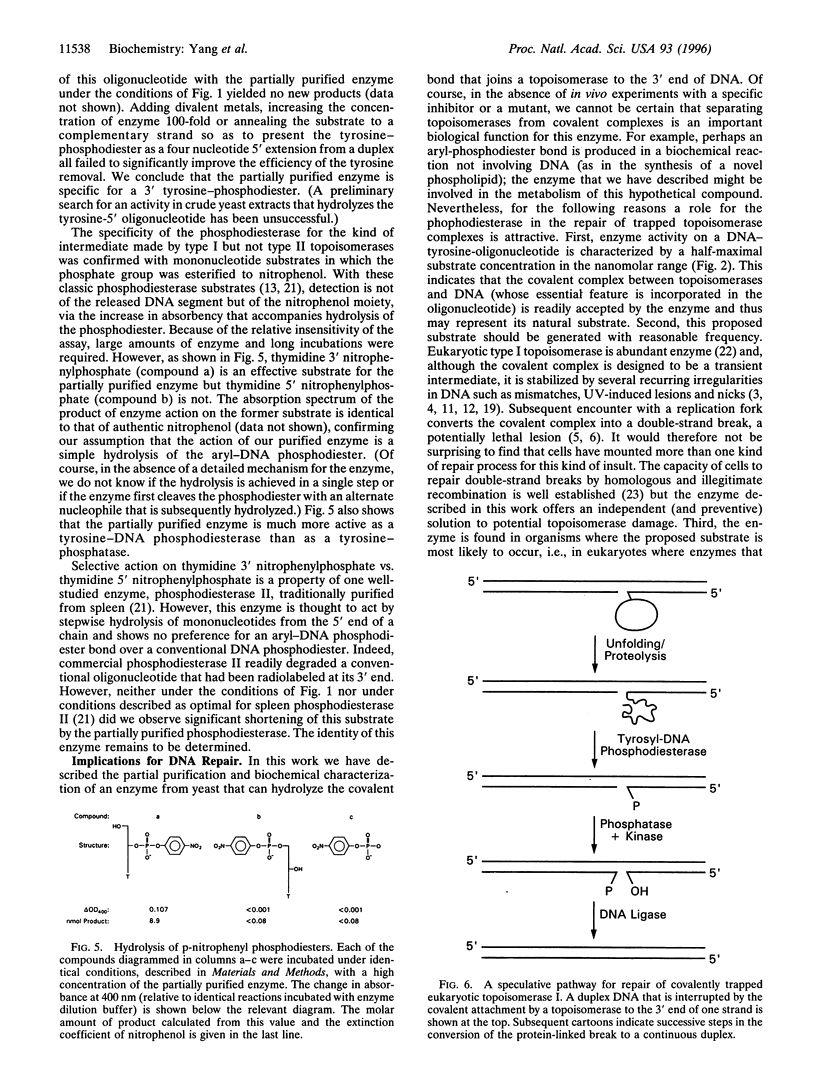
Abstract
The covalent joining of topoisomerases to DNA is normally a transient step in the reaction cycle of these important enzymes. However, under a variety of circumstances, the covalent complex is converted to a long-lived or dead-end product that can result in chromosome breakage and cell death. We have discovered and partially purified an enzyme that specifically cleaves the chemical bond that joins the active site tyrosine of topoisomerases to the 3' end of DNA. The reaction products made by the purified enzyme on a variety of model substrates indicate that the enzyme cleanly hydrolyzes the tyrosine-DNA phosphodiester linkage, thereby liberating a DNA terminated with a 3' phosphate. The wide distribution of this phosphodiesterase in eukaryotes and its specificity for tyrosine linked to the 3' end but not the 5' end of DNA suggest that it plays a role in the repair of DNA trapped in complexes involving eukaryotic topoisomerase I.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aris J. P., Blobel G. Isolation of yeast nuclei. Methods Enzymol. 1991;194:735–749. doi: 10.1016/0076-6879(91)94056-i. [DOI] [PubMed] [Google Scholar]
- Bardwell A. J., Bardwell L., Tomkinson A. E., Friedberg E. C. Specific cleavage of model recombination and repair intermediates by the yeast Rad1-Rad10 DNA endonuclease. Science. 1994 Sep 30;265(5181):2082–2085. doi: 10.1126/science.8091230. [DOI] [PubMed] [Google Scholar]
- Burgin A. B., Jr, Huizenga B. N., Nash H. A. A novel suicide substrate for DNA topoisomerases and site-specific recombinases. Nucleic Acids Res. 1995 Aug 11;23(15):2973–2979. doi: 10.1093/nar/23.15.2973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgin A. B., Jr, Nash H. A. Symmetry in the mechanism of bacteriophage lambda integrative recombination. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9642–9646. doi: 10.1073/pnas.89.20.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champoux J. J., McCoubrey W. K., Jr, Been M. D. DNA structural features that lead to strand breakage by eukaryotic type-I topoisomerase. Cold Spring Harb Symp Quant Biol. 1984;49:435–442. doi: 10.1101/sqb.1984.049.01.049. [DOI] [PubMed] [Google Scholar]
- Chen A. Y., Liu L. F. DNA topoisomerases: essential enzymes and lethal targets. Annu Rev Pharmacol Toxicol. 1994;34:191–218. doi: 10.1146/annurev.pa.34.040194.001203. [DOI] [PubMed] [Google Scholar]
- Christiansen K., Knudsen B. R., Westergaard O. The covalent eukaryotic topoisomerase I-DNA intermediate catalyzes pH-dependent hydrolysis and alcoholysis. J Biol Chem. 1994 Apr 15;269(15):11367–11373. [PubMed] [Google Scholar]
- Costin D., Potmesil M. Preclinical and clinical development of camptothecins. Adv Pharmacol. 1994;29B:51–72. doi: 10.1016/s1054-3589(08)61131-x. [DOI] [PubMed] [Google Scholar]
- Froelich-Ammon S. J., Osheroff N. Topoisomerase poisons: harnessing the dark side of enzyme mechanism. J Biol Chem. 1995 Sep 15;270(37):21429–21432. doi: 10.1074/jbc.270.37.21429. [DOI] [PubMed] [Google Scholar]
- Goldwasser F., Bae I., Valenti M., Torres K., Pommier Y. Topoisomerase I-related parameters and camptothecin activity in the colon carcinoma cell lines from the National Cancer Institute anticancer screen. Cancer Res. 1995 May 15;55(10):2116–2121. [PubMed] [Google Scholar]
- Habraken Y., Verly W. G. Chromatin 3'-phosphatase/5'-OH kinase cannot transfer phosphate from 3' to 5' across a strand nick in DNA. Nucleic Acids Res. 1986 Oct 24;14(20):8103–8110. doi: 10.1093/nar/14.20.8103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang J., Hwong C. L. Cellular regulation of mammalian DNA topoisomerases. Adv Pharmacol. 1994;29A:167–189. doi: 10.1016/s1054-3589(08)60545-1. [DOI] [PubMed] [Google Scholar]
- Kauh E. A., Bjornsti M. A. SCT1 mutants suppress the camptothecin sensitivity of yeast cells expressing wild-type DNA topoisomerase I. Proc Natl Acad Sci U S A. 1995 Jul 3;92(14):6299–6303. doi: 10.1073/pnas.92.14.6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landy A., Ross W. Viral integration and excision: structure of the lambda att sites. Science. 1977 Sep 16;197(4309):1147–1160. doi: 10.1126/science.331474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanza A., Tornaletti S., Rodolfo C., Scanavini M. C., Pedrini A. M. Human DNA topoisomerase I-mediated cleavages stimulated by ultraviolet light-induced DNA damage. J Biol Chem. 1996 Mar 22;271(12):6978–6986. doi: 10.1074/jbc.271.12.6978. [DOI] [PubMed] [Google Scholar]
- Nitiss J., Wang J. C. DNA topoisomerase-targeting antitumor drugs can be studied in yeast. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7501–7505. doi: 10.1073/pnas.85.20.7501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nunes-Düby S. E., Matsumoto L., Landy A. Site-specific recombination intermediates trapped with suicide substrates. Cell. 1987 Aug 28;50(5):779–788. doi: 10.1016/0092-8674(87)90336-9. [DOI] [PubMed] [Google Scholar]
- Pan G., Luetke K., Juby C. D., Brousseau R., Sadowski P. Ligation of synthetic activated DNA substrates by site-specific recombinases and topoisomerase I. J Biol Chem. 1993 Feb 15;268(5):3683–3689. [PubMed] [Google Scholar]
- Pommier Y., Leteurtre F., Fesen M. R., Fujimori A., Bertrand R., Solary E., Kohlhagen G., Kohn K. W. Cellular determinants of sensitivity and resistance to DNA topoisomerase inhibitors. Cancer Invest. 1994;12(5):530–542. doi: 10.3109/07357909409021413. [DOI] [PubMed] [Google Scholar]
- Pommier Y., Tanizawa A., Kohn K. W. Mechanisms of topoisomerase I inhibition by anticancer drugs. Adv Pharmacol. 1994;29B:73–92. doi: 10.1016/s1054-3589(08)61132-1. [DOI] [PubMed] [Google Scholar]
- Prinos P., Slack C., Lasko D. D. 5'phosphorylation of DNA in mammalian cells: identification of a polymin P-precipitable polynucleotide kinase. J Cell Biochem. 1995 May;58(1):115–131. doi: 10.1002/jcb.240580114. [DOI] [PubMed] [Google Scholar]
- Segall A. M., Goodman S. D., Nash H. A. Architectural elements in nucleoprotein complexes: interchangeability of specific and non-specific DNA binding proteins. EMBO J. 1994 Oct 3;13(19):4536–4548. doi: 10.1002/j.1460-2075.1994.tb06775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svejstrup J. Q., Christiansen K., Andersen A. H., Lund K., Westergaard O. Minimal DNA duplex requirements for topoisomerase I-mediated cleavage in vitro. J Biol Chem. 1990 Jul 25;265(21):12529–12535. [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1996;65:635–692. doi: 10.1146/annurev.bi.65.070196.003223. [DOI] [PubMed] [Google Scholar]
- Yeh Y. C., Liu H. F., Ellis C. A., Lu A. L. Mammalian topoisomerase I has base mismatch nicking activity. J Biol Chem. 1994 Jun 3;269(22):15498–15504. [PubMed] [Google Scholar]