Abstract
Objective : Reactive oxygen species (ROS) are a major contributing factor in diseases pathophysiology in critically ill patients. Oxidative stress usually occurs in critical illnesses, specifically during sepsis, and organ dysfunction. The anti-oxidative properties of probiotics may serve as a defense in intestine and overcome various oxidative stresses. The aim of this trial was to determine the effect of probiotics on inflammation, antioxidant capacity and lipid peroxidation in critically ill patients.
Methodology : Forty patients admitted to the intensive care unit were enrolled in this double-blind, randomized controlled trial. They were randomized to receive placebo or probiotic for 7 days. Serum levels of Total Antioxidant Capacity (TAC), Malodialdehyde (MDA), C-Reactive Protein (CRP) and Acute Physiology and Chronic Health Evaluation (APACHE II) score were measured before initiation of the study and on the 7th day.
Results: There was a significant difference in CRP levels and APACHE II score between two groups at the end of the study (P= 0.003 and 0.001, respectively). There was not a significant difference in levels of TAC and MDA between two groups.
Conclusions: Administration of probiotics to critically ill patients caused reduction in inflammation and improvement of clinical outcome. However, there were not significant changes in markers of oxidative stress.
Key Words: Probiotic, Critically ill, Oxidative stress, Sepsis
INTRODUCTION
Reactive oxygen species (ROS) are a major contributing factor of diseases in critically ill patients.1 Oxidative stress is the imbalance between ROS production and the body’s defense system. Scavengers of ROS and antioxidants are important in treating and preventing the damage caused by oxidative stress.2,3 Critically ill patients can have increased level of ROS or decreased antioxidant defenses. Several studies show that oxidative stress occurs in critical illnesses, specifically in patients with sepsis and organ dysfunction4,5 as ROS can stimulate the inflammatory system. Critical illness is associated with massive oxidative stress resulting in exacerbation of organ injury and poor clinical outcome.6 The severity of illness by APACHE III has been proportionally related to the degree of oxidative stress.7 Sepsis and multiple organ dysfunction syndrome (MODS) are the most common cause of death in critically ill patients.8 Destruction of intestinal barrier function and increased translocation of bacteria or their toxins into systemic blood flow can lead to risk of infection and MODS in critically ill patients.9,10
Nowadays the role of inflammation and oxidative stress in the pathogenesis of sepsis is obvious.11 ROS generation plays a key role in survival for septic patients.12Numerous studies have reported severe oxidative stress in patients with SIRS and sepsis, with reduced total antioxidant capacity (TAC)13,14 and increased levels of Thiobarbituric acid reactive substances15 and malondialdehyde (MDA).14,15 TAC gives information about all of the antioxidants in the organism, while MDA is a lipid peroxidation marker used to assess lipid peroxidation due to increased oxidative stress.16
Various therapies including antioxidants especially selenium17,18 have been evaluated in sepsis but none of them has been effective on survival of patients.19 Probiotics are “live microorganisms which when administered in adequate amounts confer a health benefit on the host”.20 The antioxidant effects of probiotics have been reported in different studies.21-23 Their anti-oxidative properties may serve as a defense in intestine and overcome various oxidative stresses23, so they can prevent or control several disease associated with oxidative stress24 and they seem to have beneficial effects in improvement of critically ill patients.
The purpose of this randomized clinical trial was to determine the effect of probiotic containing lactobacillus, bifidobacterium and streptococcus thermophiluson inflammation, antioxidant capacity and lipid peroxidation in critically ill patients in the intensive care unit.
METHODOLOGY
Patients admitted between December of 2011 and October of 2012 to the ICU of the Shohada Hospital (Tabriz, East Azerbaijan, Iran) were eligible for the study. After approval of ethics committee of Tabriz University of Medical Sciences and obtaining informed consent of the patients or their legal guardian, 40 patients were enrolled in this trial. The clinical trial of the study was registered in Iranian Registry of Clinical Trials with code number of (IRCT201112143320N6).
This is a report of the data base from PhD thesis entitled “Effect of probiotic containing lactobacillus, bifidobacterium and streptococusthermophilus administration on inflammatory, coagulation and oxidative factors in critically ill patients with risk of sepsis”. A total sample size of 40 subjects was calculated based on the published levels of IL-6 differences in critically ill patients25 at the 5% significance level with a power of 80%.The formula used for sample size calculation was as: n=[2(SD2) (Z α/2 + Z β ) 2] / ∆2 , which SD was 50 and ∆ was 50. Using the formula, a sample size of 15.7 for each group was achieved which regarding the loss to follow, we included 20 patients in each group.
Inclusion criteria were critically ill patients admitted to the surgical ICU with positive SIRS and APACHE II score of 15 to 30, aging 18 to 40 years old and receiving enteral nutrition who were expected to stay in ICU for at least 7 days. Exclusion criteria were pregnant and lactating women, patients who cannot tolerate enteral nutrition, those with unstable hemodynamic, immune disorders, intestinal obstruction or ischemia, cancer and patients who were expected to expire in the next 24 hours. All patients received enteral nutrition with Fresubin original fibre (Fresenius Kabi, Homburg, Germany) at the first 24-hours of the admission via nasogastric tube. It was started at 25mL/h and increased by 25 mL/h every 4 h until the target rate was achieved. Weight and height of the patients were recorded and body mass index was calculated by the formula weight (in kg)/height2 (in m). Energy requirements were calculated as 25–30 kcal/kg and protein requirements as 1.2–1.5 g/kg.
The patients were randomly assigned into two 20-person groups; the first group received standard treatment plus placebo and the second group received standard treatment plus VSL#3 (VSL Pharmaceuticals, Ft Lauderdale, FL), 2 sachets daily for 7 days. Each sachet of probiotics contained 900 billion viable lyophilized bacteria consisting of 4 strains of Lactobacillus (L. casei, L. plantarum, L. acidophilus, and L. delbrueckiisubsp. Bulgaricus), 3 strains of Bifidobacterium (B. longum, B. breve, and B. infantis) and Streptococcus salivarius subsp. Thermophilus. Blood was obtained from each patient before the study and on day 7 to evaluate TAC, MDA and CRP. TAC levels were measured using an ELISA kit (ImAnOx, Immundiagnoctic AG, Bensheim, Germany) and MDA levels were measured based on reaction with thiobarbituric acid and a spectrophotometric Assay. APACHE II score was also determined for all patients at baseline and on the 7th day. Data were analyzed by SPSS 16. P values<0.05 were considered significant for all statistical tests.
RESULTS
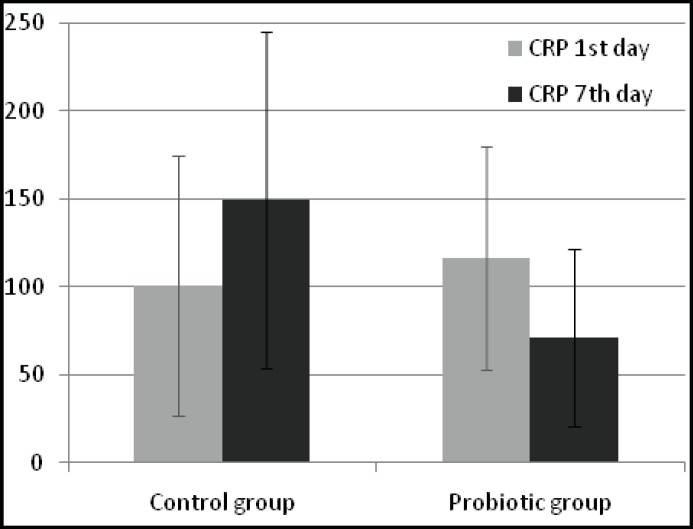
There were 20 patients in probiotic group and 20 patients in control group. No significant differences in demographic data of patients were observed between two groups (Table-I). Levels of TAC, MDA, CRP and APACHE II at the baseline and at the end of the study are shown in Table-II.TAC has significantly increased and MDA has significantly decreased in both groups, however there isn't any significant difference in their levels between two groups after the treatment (P=0.062 and 0.123, respectively). Levels of CRP (Fig.1) and APACHE II score were significantly lower in the probiotic group compared to controls at day 7 (P=0.003 and 0.001, respectively).
Table-I.
Demographic data of patients
| Control group | Probiotic group | P value | |
|---|---|---|---|
| Age (year) | 35.60±5.03* | 33.60±5.50 | 0.238 |
| Male/female | 14/6 | 13/7 | 0.500 |
| BMI | 24.70±3 | 24.30±2.92 | 0.677 |
*mean±SD BMI: Body Mass Index
Table-II.
Levels of TAC, MDA, CRP and APACHE II at baseline and at the end of the study
| Control group | Probiotic group | P value | ||
|---|---|---|---|---|
| TAC(μmol/L) | 1st day | 122.43±53.50* | 96.67±38.21 | 0.088† |
| 7th day | 153.55±73.25 | 191.95±51.28 | 0.062 | |
| MDA(nmol/ml) | 1st day | 3.28±1.58 | 4.02±1.64 | 0.159 |
| 7th day | 2.52±1.19 | 1.90±1.29 | 0.123 | |
| CRP(mg/l) | 1st day | 100.57±73.81 | 116.38±63.25 | 0.471 |
| 7th day | 149.56±95.83 | 71.20±50.43 | 0.003 | |
| APACHE | 1st day | 22.45±4.57 | 22.80±4.73 | 0.813 |
| 7th day | 20.85±7.55 | 13.85±4.82 | 0.001 |
*mean±SD †Independent-samples T test
Fig.1.
Mean (±SD) CRP levels in two groups of patients. *Patients in the probiotic group had significantly lower CRP levels by day 7 (P =0.006
DISCUSSION
The present study used a double-blind, placebo-controlled, randomized design to determine the effects of probiotics on markers of oxidative stress and inflammation in critically ill, enterally fed patients. Overall, the patients who received probiotic showed a greater reduction in inflammation than did the patients who received placebo.CRP is an acute-phase protein produced by the liver and by endothelial cells.26 It is commonly used as a marker of systemic inflammation27 and serum levels provide a useful indicator of the extent of an inflammatory process.28 CRP inhibits the production of proinflammatory cytokines and chemokines.29 In the present study patients receiving probiotic showed a reduction in CRP concentrations over the treatment. A similar finding was reported by Kotzampassi et al, who showed that a combination regimen of pro- and prebiotics (Synbiotic 2000 Forte) caused a significant reduction in CRP levels compared to placebo-treated group.30 Interestingly, in a study by Alberda et al which critically ill patients were assigned to receive viable probiotics (VSL#3), equivalent probiotic sonicates or placebo, although most of the patients showed a reduction in CRP concentrations over the treatment period, patients receiving viable probiotics had a lesser decline in CRP concentrations than patients receiving either placebo or bacterial sonicates.31
In another study by McNaught et al, enteral administration of ProViva, an oatmeal-based drink containing Lactobacillus plantarum 299v to critically ill patients had no significant effect on serum CRP levels.25 The type of probiotic used in the studies may be an explanation for these dissimilar findings.VSL#3 is a potent probiotic medical food that delivers the highest available concentration of beneficial live bacteria of any other probiotic and contains 8 different strains of live lactic acid bacteria specially selected to produce an optimal synergistic composition of bacteria.32,33 Although the type of the probiotic used in the study of Alberda et al30 was the same as the one we used and even duration of the treatment was the same in both studies (7 days), the different results seen for the CRP can be due to the patients' population. Our trial was performed in a surgical ICU which most of them were traumatic patients, so heterogeneity which is one the most important problems in ICU population was decreased.
APACHE II score which is a measurement of disease severity is closely correlated with the risk of many common diseases and hospital death.34 In our study, APACHE II score significantly decreased in probiotic group after 7 days of treatment. However, in a study performed on severe traumatic brain-injured patients with Glasgow Coma Scale scores between 5 and 8, APACHE score was not significantly affected by probiotic treatment.35The improvement in clinical outcome shown by a significant decrease in APACHE II score in this study can be related to the reduction of inflammation by the use of probiotics.
Of the many biological targets of oxidative stress, lipids are the most involved class of bio-molecules. Lipid oxidation gives rise to a number of secondary products. Malondialdehyde (MDA) is the principal and most studied product of polyunsaturated fatty acid peroxidation.36 TAC had a significant increase and MDA had a significant decrease in both groups at the end of the study, which can be due to the usage of the various antioxidant drugs in the standard treatment of the patients in intensive care unit. Nevertheless, there was not a significant difference between two groups after 7 days of treatment with probiotics. However, the increase of TAC is more in probiotic group compared to control group which shows that body's antioxidant capacity increases with the use of probiotics and according to the P value (P= 0.062), it seems that significant results could be achieved by increasing sample size. Although different studies have shown the anti-oxidative effects of probiotics21-24, to the best of our knowledge, no study has been performed regarding the effect of probiotic administration on markers of oxidative stress in critically ill patients.
Limitation of the study: This is a single center study with 40 patients included, so we need future multi center trials with larger sample size of critically ill patients. This trial was performed in surgical patients, so for routine usage of probiotics in critically ill patients, they should be examined in medical or mixed type ICUs. There is also need to trials that define the best dosage and optimal duration of therapy in these patients.
CONCLUSION
In conclusion, the results of this randomized trial suggest that administration of probiotics in critically ill patients is associated with clinical benefits compared to placebo-treated patients: they significantly reduce the levels of CRP and APACHE score. However, they did not significantly affect the levels of the markers of oxidative stress. So, further studies with larger sample size are needed to clarify their usefulness in this group of patients.
Authors' contribution:
ME: Study design, revising manuscript. SS: Study design, sample collection and preparing manuscript draft. AM: Study design, sample collection and revising manuscript. HH: Sample analyzing via commercially available enzyme-linked immunosorbent assay kit.
ACKNOWLEDGMENT
This is a report of data base from PhD thesis entitled “Effect of probiotic containing lactobacillus, bifidobacterium and streptococusthermophilus administration on inflammatory, coagulation and oxidative factors in critically ill patients with risk of sepsis” registered in Students Research Committee of Tabriz University of Medical Sciences. We acknowledge VSL Pharmaceuticals, Inc, Sigma-Tau Pharmaceuticals, Inc. for preparation of VSL#3 and placebos. We would like to express our special thanks to the Mr. Shahrokh Teshnedel, Mr. Qorbanali Tarinezhad and staff of Shohada Hospital ICU.
References
- 1.Goodyear-Bruch C, Pierce JD. Oxidative stress in critically ill patients. Am J Crit Care. 2002;11:543–551. [PubMed] [Google Scholar]
- 2.Gutteridge J, Mitchell J. Redox imbalance in the critically ill. Br Med Bull. 1999;55:49–75. doi: 10.1258/0007142991902295. [DOI] [PubMed] [Google Scholar]
- 3.Supinski G. Free radical induced respiratory muscle dysfunction. Mol Cell Biochem. 1998;179:99–110. doi: 10.1023/a:1006859920875. [DOI] [PubMed] [Google Scholar]
- 4.Oldham KM, Bowen PE. Oxidative stress in critical care: is antioxidant supplementation beneficial? J Am Diet Assoc. 1998;98(9):1001–1008. doi: 10.1016/S0002-8223(98)00230-2. [DOI] [PubMed] [Google Scholar]
- 5.Schorah CJ, Downing C, Piripitsi A, Gallivan L, Al-Hazaa AH, Sanderson MJ, et al. Total vitamin C, ascorbic acid, and dehydroascorbic acid concentrations in plasma of critically ill patients. Am J ClinNutr. 1996;63:760–765. doi: 10.1093/ajcn/63.5.760. [DOI] [PubMed] [Google Scholar]
- 6.Crimi E, Sica V, Williams-Ignarro S, Zhang H, Slutsky AS, Ignarro LJ, et al. The role of oxidative stress in adult critical care. Free Rad Biol Med. 2006;40:398–406. doi: 10.1016/j.freeradbiomed.2005.10.054. [DOI] [PubMed] [Google Scholar]
- 7.Alonso de Vega JM, Díaz J, Serrano E, Carbonell LF. Plasma redox status relates to severity in critically ill patients. Crit Care Med. 2000;28:1812–1814. doi: 10.1097/00003246-200006000-00021. [DOI] [PubMed] [Google Scholar]
- 8.Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, Pinsky MR. Epidemiology of severe sepsis in the United States: analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 9.MacFieJ , O’Boyle C, Mitchell CJ, Buckley PM, Johnstone D, Sudworth P. Gut origin of sepsis: a prospective study investigating associations between bacterial translocation, gastric microflora, and septic morbidity. Gut. 1999;45(2):223–228. doi: 10.1136/gut.45.2.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.MacFie J. Current status of bacterial translocation as a cause of surgical sepsis. Br Med Bull. 2004;71:1–11. doi: 10.1093/bmb/ldh029. [DOI] [PubMed] [Google Scholar]
- 11.Eslami K, Mahmoodpoor A, Ahmadi A, Abdollahi M, Kamali K, Mousavi S, et al. Positive effect of septimebTM on mortality rate in severe sepsis: a novel non antibiotic strategy. DARU J Pharm Sci. 2012:20–40. doi: 10.1186/2008-2231-20-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Galley HF, Davies MJ, Webster NR. Xanthine oxidase activity and free radical generation in patients with sepsis syndrome. Crit Care Med. 1996;24:1649–1653. doi: 10.1097/00003246-199610000-00008. [DOI] [PubMed] [Google Scholar]
- 13.Tsai K, Hsu T, Kong C, Lin K, Lu F. Is the endogenous peroxylradical scavenging capacity of plasma protective in systemic inflammatory disorders in humans? Free Radic Biol Med. 2000;28:926–933. doi: 10.1016/s0891-5849(00)00180-5. [DOI] [PubMed] [Google Scholar]
- 14.Alonso de Vega JM, Diaz J, Serrano E, Carbonell LF. Oxidative stress in critically ill patients with systemic inflammatory response syndrome. Crit Care Med. 2002;30:1782–1786. doi: 10.1097/00003246-200208000-00018. [DOI] [PubMed] [Google Scholar]
- 15.Goode HF, Cowley HC, Walker BE, Howdle PD, Webster NR. Decreased antioxidant status and increased lipid peroxidation in patients with septic shock and secondary organ dysfunction. Crit Care Med. 1995;23:645–646. doi: 10.1097/00003246-199504000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Torun AN, Kulaksizoglu S, Kulaksizoglu M, Pamuk BO, Isbilen E, Tutuncu NB. Serum total antioxidant status and lipid peroxidation marker malondialdehyde levels in overt and subclinical hypothyroidism. ClinEndocrinol (Oxf) 2009;70(3):469–474. doi: 10.1111/j.1365-2265.2008.03348.x. [DOI] [PubMed] [Google Scholar]
- 17.Angstwurm MW, Engelmann L, Zimmermann T, Lehmann C, Spes CH, Abel P, et al. Selenium in Intensive Care (SIC): results of a prospective randomized, placebo-controlled, multiple-center study in patients with severe systemic inflammatory response syndrome, sepsis, and septic shock. Crit Care Med. 2007;35:118–126. doi: 10.1097/01.CCM.0000251124.83436.0E. [DOI] [PubMed] [Google Scholar]
- 18.Valenta J, Brodska H, Drabek T, Hendl J, Kazda A. High-dose selenium substitution in sepsis: a prospective randomized clinical trial. Intensive Care Med. 2011;37:808–815. doi: 10.1007/s00134-011-2153-0. [DOI] [PubMed] [Google Scholar]
- 19.Mahmoodpoor A, Eslami K, Mojtahedzadeh M, Najafi A, Ahmadi A, Dehnadi-Moghaddam A, et al. Examination of Setarud (IMOD™) in the management of patients with severe sepsis. DARU J Pharm Sci. 2010;18:23–28. [PMC free article] [PubMed] [Google Scholar]
- 20.Food and Agriculture Organization (FAO) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria: report of a joint FAO/WHO expert consultation on evaluation of health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. Geneva: Food and Agriculture Organization; 2001. [Google Scholar]
- 21.Lin MY, Yen CL. Antioxidative ability of lactic acid bacteria. J Agric Food Chem. 1999;47(4):1460–1466. doi: 10.1021/jf981149l. [DOI] [PubMed] [Google Scholar]
- 22.Lin MY, Chang FY. Antioxidative effect of intestinal bacteria Bifidobacterium longumATCC15708 and Lactobacillus acidophilus ATCC 4356. Dig Dis Sci. 2000;45:1617–1622. doi: 10.1023/a:1005577330695. [DOI] [PubMed] [Google Scholar]
- 23.Kullisaar T, Zilmer M, Mikelsaar M, Vihalemm T, Annuk H, Kairane C, et al. Two antioxidative lactobacilli strains as promising probiotics. Int J Food Microbiol. 2002;72:215–224. doi: 10.1016/s0168-1605(01)00674-2. [DOI] [PubMed] [Google Scholar]
- 24.Amaretti A, Di Nunzio M, Pompei A, Raimondi S, Rossi M, Bordoni A. Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol. 2012 doi: 10.1007/s00253-012-4241-7. (Epub) [DOI] [PubMed] [Google Scholar]
- 25.McNaught CE, Woodcock NP, Anderson AD, MacFie J. A prospective randomised trial of probiotics in critically ill patients. ClinNutr. 2005;24:211–219. doi: 10.1016/j.clnu.2004.08.008. [DOI] [PubMed] [Google Scholar]
- 26.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, et al. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 27.Ballou SP, Kushner I. C-reactive protein and the acute phase response. Adv Intern Med. 1992;37:313–336. [PubMed] [Google Scholar]
- 28.Sheeran P, Hall GM. Cytokines in anaesthesia. Br J Anaesth. 1997;78:201–219. doi: 10.1093/bja/78.2.201. [DOI] [PubMed] [Google Scholar]
- 29.Szalai AJ, Nataf S, Hu XZ, Barnum SR. Experimental allergic encephalomyelitis is inhibited in transgenic mice expressing human C-reactive protein. J Immunol. 2002;168:5792–5797. doi: 10.4049/jimmunol.168.11.5792. [DOI] [PubMed] [Google Scholar]
- 30.Kotzampassi K, Giamarellos-Bourboulis EJ, Voudouris A, Kazamias P, Eleftheriadis E. Benefits of a symbiotic formula (Synbiotic 2000Forte) in critically ill trauma patients: early results of a randomized controlled trial. World J Surg. 2006;30(10):1848–1855. doi: 10.1007/s00268-005-0653-1. [DOI] [PubMed] [Google Scholar]
- 31.Alberda C, Gramlich L, Meddings J, Field C, McCargar L, Kutsogiannis D, et al. Effects of probiotic therapy in critically ill patients: a randomized, double-blind, placebo-controlled trial. Am J ClinNutr. 2007;85:816–823. doi: 10.1093/ajcn/85.3.816. [DOI] [PubMed] [Google Scholar]
- 32.Brigidi P, Vitali B, Swennen E, Bazzocchi G, Matteuzzi D. Effects of probiotic administration upon the composition and enzymatic activity of human fecal microbiota in patients with irritable bowel syndrome or functional diarrhea. Res Microbiol. 2001;52:735–741. doi: 10.1016/s0923-2508(01)01254-2. [DOI] [PubMed] [Google Scholar]
- 33.Venturi A, Gionchetti P, Rizzello F, Johansson R, Zucconi E, Brigidi P, et al. Impact on the composition of the faecal flora by a new probiotic preparation: preliminary data on maintenance treatment of patients with ulcerative colitis. Aliment Pharmacol Ther. 1999;13(8):1103–1108. doi: 10.1046/j.1365-2036.1999.00560.x. [DOI] [PubMed] [Google Scholar]
- 34.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13:818–829. [PubMed] [Google Scholar]
- 35.Tan M, Zhu JC, Du J, Zhang LM, Yin HH. Effects of probiotics on serum levels of Th1/Th2 cytokine and clinical outcomes in severe traumatic brain-injured patients: a prospective randomized pilot study. Crit Care. 2011;15:290. doi: 10.1186/cc10579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Rio D, Stewart AJ, Pellegrini N. A review of recent studies on malondialdehyde as toxic molecule and biological marker of oxidative stress. Nutr Metab Cardiovasc Dis. 2005;15(4):316–328. doi: 10.1016/j.numecd.2005.05.003. [DOI] [PubMed] [Google Scholar]