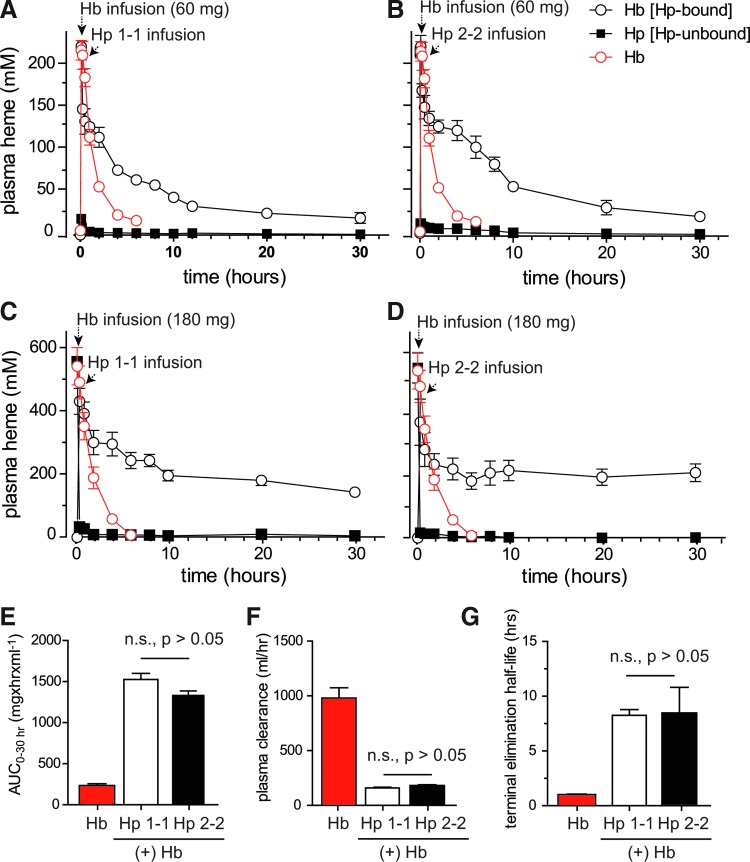
FIG. 5.
Extravascular decompartmentalization of Hb, Hp1-1, and Hp2-2. Plasma concentration versus time curves of plasma Hb (expressed as heme concentration). (A) Hemoglobin infusion (60 mg) followed by Hp1-1 (60 mg) infused at 10 min post-Hb infusion. (B) Hemoglobin infusion (60 mg) followed by Hp2-2 (60 mg) infused at 10 min post-Hb infusion. (C) Hemoglobin infusion (180 mg) followed by Hp1-1 (180 mg) infused at 10 min post-Hb infusion. (D) Hemoglobin infusion (180 mg) followed by Hp2-2 (180 mg) infused at 10 min post-Hb infusion. In both panels, black and white open circles and dark squares represent bound and free Hb, respectively, whereas open circles represent the plasma concentration–time curve of Hb alone. Comparison of primary pharmacokinetic data from the 60 mg Hb exposure group (E) AUC0–4h Hb, AUC0–30h Hb:Hp1-1 and Hb:Hp2-2. (F) Plasma clearance (dose/AUC) based on Hb AUC0–4h, and Hb:Hp AUC0–30h. (G) Terminal elimination half-life based on the negative value of the terminal slope (k) of the log-linear plasma concentration–time curve for Hb, Hb:Hp1-1, and Hb:Hp2-2. Parameters were all significantly different (p<0.001) between Hb and Hb:Hp complexes, but not between Hb:Hp1-1 and Hb:Hp2-2 based on a nonparametric analyses (Kruskal–Wallis test followed by a Dunns post-test for group comparisons). To see this illustration in color, the reader is referred to the web version of this article at www.liebertpub.com/ars