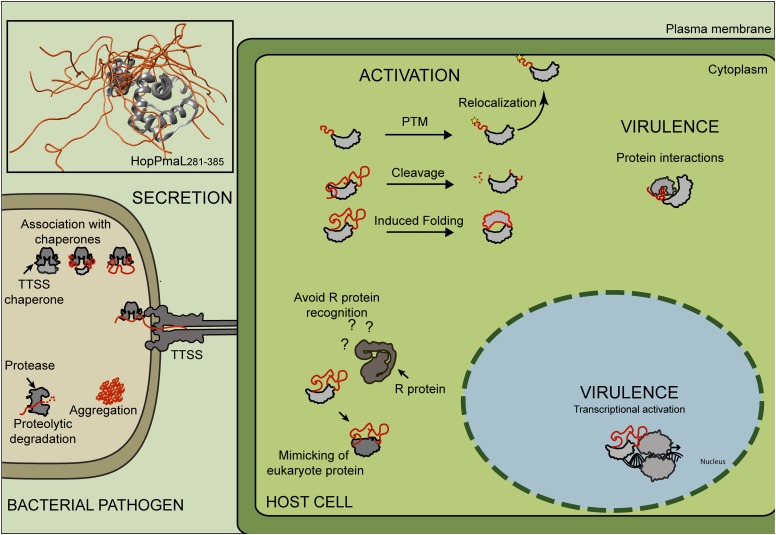
Figure 1.
Intrinsic Disorder in Effector Proteins.
Bacterial effector proteins are secreted via TTSS into the host cell cytoplasm, where they manipulate host cell immune signaling and physiology. Structural flexibility is required for efficient secretion via TTSS, as proteins can only be secreted in an unfolded state. Posttranslational modifications (PTM) can be added to residues within ID segments to determine subcellular localization of the protein inside the host cell. This structural signature may also contribute to effector virulence via mimicking or interactions with signaling proteins and evasion of immune recognition by Resistance (R) proteins. Top left box: The structural model of HopPmaL281-385 (PDBID: 2LF3; inset top left corner) illustrates the structural flexibility of ID regions. The unfolded region is depicted in orange.