Figure 8.
Spectroscopy Analysis of Native Proteins Purified from Deriphat-PAGE and Recombinant LHCSR.
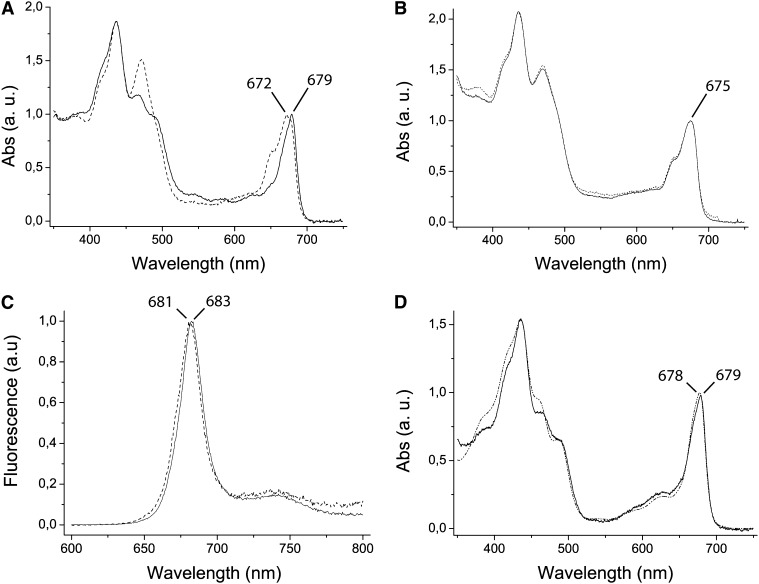
(A) Absorption spectrum of fraction 1 eluted from the gel. Sample containing LHCSR protein from thylakoids of excess light–treated wild-type P. patens plants (continuous line) is compared with the corresponding fraction purified from lhcsr KO solubilized thylakoids (pointed line). The LHCSR-containing fractions from the wild type had absorption spectra strongly contrasting with those from lhcsr KO thylakoids. a.u., arbitrary units.
(B) Absorption spectrum of fraction 5, corresponding to LHC monomers of the wild type (continuous line) and lhcsr KO (pointed line).
(C) Fraction 1 fluorescence emission at 300K with excitation at 440 nm of the wild type (continuous line) and lhcsr KO (pointed line).
(D) Absorption spectrum of fraction 1 eluted from the gel and corrected for scattering. Sample containing LHCSR protein from thylakoids of wild-type excess light–treated P. patens plants (continuous line) is compared with the spectrum of recombinant LHCSR3 from C. reinhardtii (broken line; redrawn from Bonente et al., 2011a).