The best-characterized endosomal sorting process is the internalization of select transmembrane proteins in clathrin-coated vesicles. The process is triggered by the recognition of signals in their cytosolic domains.
Abstract
The endosomal system is expansive and complex, characterized by swift morphological transitions, dynamic remodeling of membrane constituents, and intracellular positioning changes. To properly navigate this ever-altering membrane labyrinth, transmembrane protein cargoes typically require specific sorting signals that are decoded by components of protein coats. The best-characterized sorting process within the endosomal system is the rapid internalization of select transmembrane proteins within clathrin-coated vesicles. Endocytic signals consist of linear motifs, conformational determinants, or covalent modifications in the cytosolic domains of transmembrane cargo. These signals are interpreted by a diverse set of clathrin-associated sorting proteins (CLASPs) that translocate from the cytosol to the inner face of the plasma membrane. Signal recognition by CLASPs is highly cooperative, involving additional interactions with phospholipids, Arf GTPases, other CLASPs, and clathrin, and is regulated by large conformational changes and covalent modifications. Related sorting events occur at other endosomal sorting stations.
The internalization of a subset of plasma membrane proteins by clathrin-mediated endocytosis is one the best-characterized sorting processes that takes place in the endomembrane system of eukaryotic cells (Kirchhausen 2014). Selection of transmembrane proteins (referred to as “cargo”) for internalization by clathrin-mediated endocytosis involves recognition of endocytic signals in the cytosolic domains of the proteins by adaptors located in the inner layer of clathrin coats. Signal–adaptor interactions lead to concentration of the transmembrane proteins within clathrin-coated pits that eventually bud into the cytoplasm as clathrin-coated vesicles (Kirchhausen 2014). Transmembrane proteins that have endocytic signals are thus rapidly delivered to endosomes, whereas those that lack signals remain at the plasma membrane. This article summarizes recent progress in the elucidation of the mechanisms of signal recognition in clathrin-mediated endocytosis, with additional reference to related intracellular sorting events. Further information on this topic can be found in previous reviews (Bonifacino and Traub 2003; Traub 2009; Kelly and Owen 2011).
DIVERSITY OF ENDOCYTIC SIGNALS
The notion that endocytic receptors possess internalization signals was initially derived from morphologic, biochemical, and genetic studies of lipoprotein receptors, well before there was any knowledge of receptor structure (Brown and Goldstein 1979). With the advent of gene cloning, the amino acid sequences of various endocytic receptors became known in steady succession. Initial sequence comparisons, however, did not reveal any conserved groups of amino acids that could constitute a common endocytic signal. The identification of endocytic signals would end up requiring extensive molecular dissection of the receptor sequences using a combination of mutational and functional analyses. This effort led to the current understanding of endocytic signals as a highly diverse set of structural features in the cytosolic domains of transmembrane proteins, which can be grouped into three functionally analogous but structurally distinct classes: (1) linear motifs, (2) conformational determinants, and (3) covalent modifications.
Linear motifs are short arrays of invariant and variant amino acids, including “tyrosine-based” YXXØ (Collawn et al. 1990; Jadot et al. 1992) and [FY]XNPX[YF] motifs (Chen et al. 1990; Collawn et al. 1991), and “dileucine-based” [DE]XXXL[LI] motifs (Table 1) (Letourneur and Klausner 1992; Pond et al. 1995). In this notation, amino acids are represented in single-letter code, X indicates any amino acid, Ø indicates an amino acid with a bulky hydrophobic side chain, and the brackets mean that either amino acid is allowed at that position. The invariant amino acids are the most critical elements, although the variant amino acids influence the strength and fine specificity of the signals. The function of the signals can also be affected by flanking sequences (Ohno et al. 1998), phosphorylation of amino acids at or near the motif (Shiratori et al. 1997; Pitcher et al. 1999), their spacing from the transmembrane domain (Rohrer et al. 1996), and palmitoylation of nearby cysteine residues (Schweizer et al. 1996). Linear motifs that function as signals are generally found within unstructured regions of the cytosolic domains.
Table 1.
Endocytic signals and adaptors
| Signals or domains | Adaptors | Adaptor subunits or domains | References |
|---|---|---|---|
| YXXØ | AP-2 | µ2 | Collawn et al. 1990; Jadot et al. 1992; Ohno et al. 1995; Owen and Evans 1998 |
| [DE]XXXL[LI] | AP-2 | α-σ2 | Letourneur and Klausner 1992; Pond et al. 1995; Chaudhuri et al. 2007; Doray et al. 2007; Kelly et al. 2008 |
| Acidic clusters | AP-2? | α? | Voorhees et al. 1995; Lindwasser et al. 2008 |
| [YF]XNPX[YF] | ARH; Dab2; Idol; SNX17, 27, and 31 | PTB domain | Chen et al. 1990; Collawn et al. 1991; He et al. 2002; Mishra et al. 2002; Stockinger et al. 2002; Zelcer et al. 2009; Ghai et al. 2013 |
| NPFX(1,2)D | Sla1p | SLA1 homology domain | Tan et al. 1996; Howard et al. 2002 |
| Ubiquitin | Eps15, Epsins 1 and 2 | UIM domain | Polo et al. 2002; Shih et al. 2002 |
| GPCR phosphorylation | β-arrestins 1 and 2 | Amino terminus | Ferguson et al. 1996; Goodman et al. 1996 |
| Synaptotagmin I C2A (C2B) domain | Stonin 2 | μHD domain | Martina et al. 2001; Walther et al. 2001 |
| Mid2p cytosolic domain | Syp1p | μHD domain | Reider et al. 2009 |
| Alk8 cytosolic domain | Fcho1 | μHD domain | Umasankar et al. 2012 |
| VAMP 7 longin domain | Hrb, AP-3 | Carboxy-terminal unstructured domain | Pryor et al. 2008; Kent et al. 2012 |
| VAMP 2, VAMP 3, VAMP 8 SNARE motifs | CALM | ANTH domain | Miller et al. 2011 |
ARH, autosomal recessive hypercholesterolemia; PTB, phosphotyrosine-binding; UIM, ubiquitin-interacting motifs; GPCR, G-protein-coupled receptor; CALM, clathrin assembly lymphoid myeloid leukemia.
Not all signals, however, are linear sequences or fit a canonical motif. There are now many examples of folded domains that contain information for endocytosis (Table 1) (Pryor et al. 2008; Yu et al. 2010; Miller et al. 2011). This information consists of conformational arrays of amino acids on the surface of the folded domains. Unlike linear motifs, which are common to many proteins, each conformational array described to date appears to be unique for a specific cargo. Finally, covalent modifications such as phosphorylation of hydroxyl amino acids (Ferguson et al. 1996; Di Fiore and von Zastrow 2014) or polyubiquitination on the ε-amino group of lysine residues (Hicke and Riezman 1996; Piper et al. 2014) in the cytosolic domains can also function as endocytic signals (Table 1). In these cases, the modifying groups do not modulate the activity of underlying linear or conformational signals, but themselves act as recognition determinants. Multiple or overlapping signals can occur within the same cytosolic domain (for example, see Johnson and Kornfeld 1992; Doray et al. 2008; Goh et al. 2010; Prabhu et al. 2012). Similar types of signal participate in sorting events that take place at intracellular compartments, such as the trans-Golgi network (TGN) and endosomes.
MULTIPLICITY OF ADAPTORS
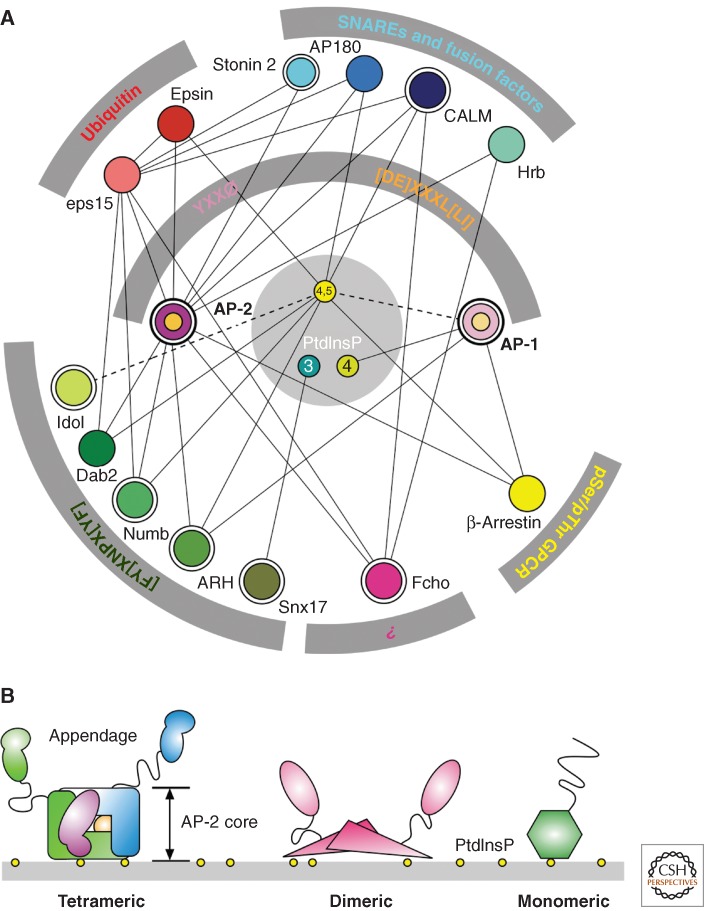
The recognition of such a wide diversity of endocytic and intracellular sorting signals obviously necessitates the existence of multiple adaptors. Indeed, many proteins located in the inner layer of protein coats—including proteins that were initially categorized as “accessory”—are now known to function as sorting adaptors (Fig. 1; Table 1). Depending on the identity of the scaffolding protein that forms the outer layer, coats are classified as clathrin coats or nonclathrin coats. Coats involved in rapid internalization from the plasma membrane contain clathrin as their main constituent and a set of adaptors known as “clathrin-associated sorting proteins” (CLASPs) (Fig. 1A). Clathrin coats containing different sets of CLASPs, as well as nonclathrin coats, mediate intracellular sorting events. CLASPs are recruited to membranes primarily via interactions with specific phosphoinositide lipids, small GTPases of the Arf family, and/or other CLASPs. Clathrin then binds to the CLASP armature and polymerizes into an overlying polyhedral scaffold. Concomitantly, CLASPs engage sorting signals in the cytosolic domains of transmembrane cargo, leading to cargo capture and stabilization of the coats. Both CLASP–clathrin (Dell'Angelica et al. 1998; Drake and Traub 2001) and CLASP–CLASP interactions (Brett et al. 2002) involve linear motifs (analogous to, but distinct from, cargo sorting signals) binding to folded domains, highlighting the general role of this binding mode in the assembly and function of clathrin coats. Most interactions among components of clathrin coats are of moderate to low affinity (typically in the 1–100 µM range), making this mechanism of sorting a highly cooperative and dynamic process.
Figure 1.
The endocytic cargo–adaptor interaction network. (A) Schematic representation of selected sorting signal-recognition partner relationships for endocytic trafficking. Both protein–protein and protein–lipid (PtdInsP) interactions among the cargo-selective machinery are highlighted with connection lines. The solid lines indicate documented physical interactions, whereas dashed lines connote either interactions possible based on properties of other domain relatives (IDOL) or known (AP-1), but still of unclear functional necessity. Adaptors, CLASPs, and regulators (Idol) discussed explicitly are double circled. (B) Representative modular domain architecture classes of selected endocytic proteins. Heterotetrameric examples are AP-1, AP-2, and AP-3; dimeric examples are Fcho1, Syp1p, and eps15; and monomeric CLASPs include ARH, Dab2, Numb, CALM, AP180, epsin, and β-arrestin. Tertiary-structured domains are indicated by geometric shapes, and intrinsically disordered protein segments by a line.
From a structural standpoint, CLASPs can be categorized as (1) oligomeric (tetrameric or dimeric), and (2) monomeric (Fig. 1B). The main endocytic adaptor is the clathrin-associated, heterotetrameric adaptor protein 2 (AP-2) complex. This complex is composed of two large “adaptin” subunits (α and β2), one medium-sized subunit (μ2), and one small subunit (σ2). AP-2 is a member of a family of homologous complexes that also includes AP-1 (γ-β1-μ1-σ1), AP-3 (δ-β3-μ3-σ3), AP-4 (ε-β4-μ4-σ4), AP-5 (ζ-β5-μ5-σ5), and COPI-F (γ-COP-β-COP-δ-COP-ζ-COP) (corresponding subunit composition in parentheses) (Robinson 2004; Hirst et al. 2011). All of these complexes are components of clathrin or nonclathrin coats that mediate sorting in intracellular compartments. They comprise a large globular “core” consisting of the amino-terminal “trunk” domains of the large subunits plus the entire medium and small subunits. The carboxy-terminal portions of the large subunits extend from the core as two long projections, each comprising a long disordered “hinge” sequence and a globular “ear” or “appendage” domain. The core mediates recruitment to membranes and sorting-signal recognition, whereas the hinge-appendage extensions interact with clathrin, other adaptors, and various accessory proteins. Dimeric and monomeric CLASPs and related adaptors consist of a single polypeptide chain in which the same functions are distributed among several globular domains joined by disordered linkers, giving them a “beads-on-a-string” appearance.
RECOGNITION OF LINEAR MOTIFS BY AP-2
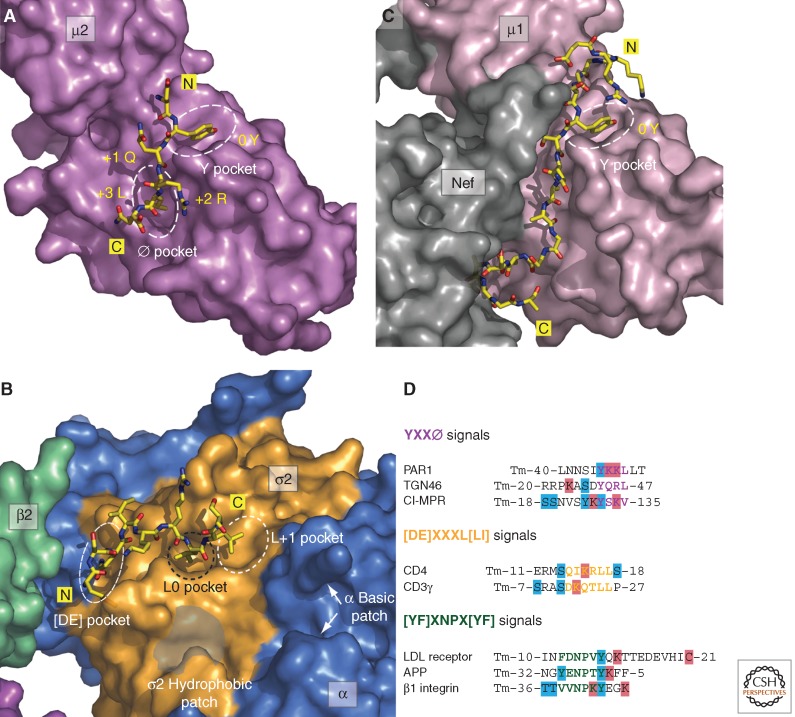
AP-2 recognizes YXXØ and [DE]XXXL[LI] signals through binding to two distinct sites on the core domain. The binding site for YXXØ motifs is located on the carboxy-terminal domain of μ2 (μ2-C) (Ohno et al. 1995), an immunoglobulin-like β-sandwich fold having hydrophobic pockets for the Y and Ø residues (Fig. 2A) (Owen and Evans 1998). The Y pocket cannot accommodate phosphotyrosine, explaining why tyrosine phosphorylation prevents binding of YXXØ motifs to μ2 (Boll et al. 1996; Ohno et al. 1996) and endocytosis (Shiratori et al. 1997). The μ2-C domain has also been shown to bind the folded DEP domain of the signaling adaptor Disheveled at a surface near the YXXØ-binding site (Yu et al. 2010). The [DE]XXXL[LI]-binding site is on the α-σ2 hemicomplex (Chaudhuri et al. 2007; Doray et al. 2007), with the acidic residue of the motif interacting with basic residues on both α and σ2, and the two hydrophobic residues fitting into adjacent hydrophobic pockets on σ2 (Fig. 2B) (Kelly et al. 2008; Mattera et al. 2011). A phosphoserine residue at position −5 from the first leucine (considered position 0) substitutes for the acidic residue at position −4 in the noncanonical dileucine signal from CD4, exemplifying how phosphorylation can also positively regulate AP-2 recognition and endocytosis (Fig. 2D) (Pitcher et al. 1999; Kelly et al. 2008).
Figure 2.
AP-1 and AP-2 cargo-binding surfaces. (A,B) Molecular details of the YXXØ and [DE]XXXL[LI] interaction surfaces on AP-2. The TGN38-derived YQRL signal cocrystalized with the AP-2 µ2-C domain (PDB ID: 1BXX) (Owen and Evans 1998) and the CD4 dileucine signal bound to the AP-2 heterotetramer core (PDB ID: 2JKR) (Kelly et al. 2008). Amino- and carboxy-terminal ends of the peptide signals (yellow) in stick representation are shown with oxygen colored red and nitrogen colored blue. The binding pockets for key anchor residues are outlined with white dashed ovals. (C) Molecular surface of the ternary AP-1 µ1 subunit•HIV-1 Nef•MHC-1 complex that stabilizes the noncanonical YXXØ signal in MHC-1 in the absence of an effective hydrophobic Ø residue. (D) Proximity of posttranslationally phosphorylated (blue) or ubiquitinated (red) residues identified within or adjacent to different primary-sequence-based sorting signals in the indicated proteins, including the protease-activated receptor 1 (PAR1), the cation-independent mannose 6-phosphate receptor (CI-MPR), and amyloid precursor protein (APP). Sorting signals are shown in color-coded bold type and the number of amino acid residues preceding or following the individual signals from the transmembrane (Tm) domain are indicated. All modifications listed in the PhosphoSitePlus mass spectrometry database (Hornbeck et al. 2004).
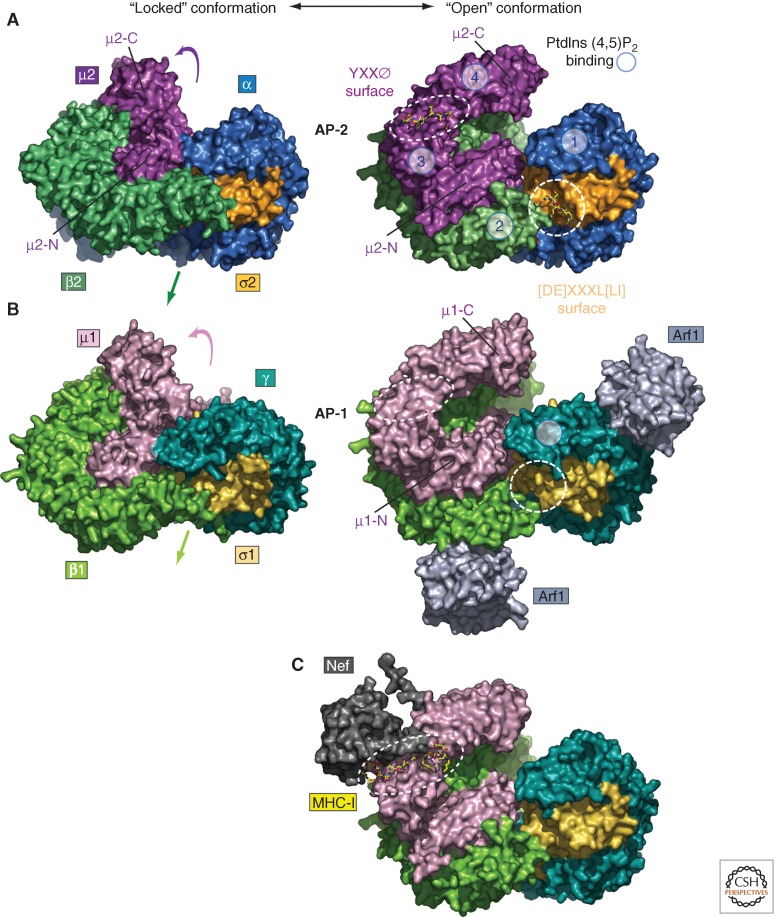
Strikingly, the AP-2 core occurs in two distinct conformations: a “locked” conformation in which the binding sites for both YXXØ and [DE]XXXL[LI] signals are occluded by regions of β2 (Collins et al. 2002), and an “open” conformation in which both sites are accessible (Fig. 3A) (Jackson et al. 2010). The change from the locked to the open conformation involves translocation of the μ2-C domain to an orthogonal face and displacement of the amino terminus of β2 from the α–σ2 interface. In the open conformation, the YXXØ- and [DE]XXXL[LI]-binding sites are coplanar with four electropositive patches that bind phosphatidylinositol 4,5-bisphosphate (PtdIns(4,5)P2) (Jackson et al. 2010), a phosphoinositide that is highly enriched in the plasma membrane (Di Paolo and De Camilli 2006). This coplanarity ensures coupling of signal recognition to membrane recruitment (Jackson et al. 2010). On the basis of these observations, it has been proposed that the AP-2 core exists in equilibrium between two conformers: the locked form that predominates in the cytosol and the open form that is mainly associated with membranes. The generation of PtdIns(4,5)P2 and availability of signal-bearing cargo at the plasma membrane shift the equilibrium toward the open form. AP-2 phosphorylation events also appear to contribute to the shift. For example, phosphorylation of a threonine residue (Thr156) in the linker that connects the amino- and carboxy-terminal domains of μ2 by the adaptor-associated kinase 1 (AAK1) enhances binding of AP-2 to endocytic signals and promotes receptor internalization (Olusanya et al. 2001; Conner and Schmid 2002; Ricotta et al. 2002). The μ2 linker is disordered in the locked form but folds into a four-turn α helix that binds to β2 in the open form (Jackson et al. 2010). Thr156 phosphorylation could thus cause stabilization of this μ2–β2 interaction and, by extension, favor the open conformation of the core. In addition, β2 residue Tyr6 undergoes phosphorylation on activation of the epidermal growth factor (EGF) receptor kinase (Huang et al. 2003). In the locked form of the AP-2 core, β2 Tyr6 occupies the σ2 hydrophobic pocket for the [LI] residue in [DE]XXXL[LI] motifs (Kelly et al. 2008). Phosphorylation of this residue could unblock the dileucine-binding site, also favoring conversion to the open form of the AP-2 core. Because internalization of the activated EGF receptor is partly dependent on a dileucine-based signal (Huang et al. 2003), this phosphorylation event illustrates how a signaling receptor can promote its own endocytosis through activation of AP-2.
Figure 3.
Allosteric-regulated exposure of the AP-1 and AP-2 cargo-binding surfaces. (A,B) Space-filling surface representations of AP-1 and AP-2 heterotetramers highlight the conformational transitions on conversion from the AP-2 “locked” state (PDB ID: 2VGL) (Collins et al. 2002) to the open state (PBD ID: 2XA7) (Jackson et al. 2010). A view of the membrane-attached face is depicted and the four subunits colored to reflect structural and functional conservation. Opening chain movements are indicated (colored arrows). Cocrystalized YXXØ and [DE]XXXL[LI] peptide signals (rendered in yellow stick representation) are delimited with white dashed circles. The relative position of the four spatially discrete PtdIns(4,5)P2 binding sites that only become coplanar on transition to the open conformation in AP-2 are circled and numbered. Similar bottom-up views of the AP-1 heteroterameric core in the closed (PDB ID: 1W63) (Heldwein et al. 2004) and open (PDB ID: 4HMY) (Ren et al. 2013) conformations, with an Arf1 GTPase contacting each large chain trunk domain. The location of the PtdIns(4)P-binding site on the γ subunit (Heldwein et al. 2004) is circled in the open conformation. (C) A composite molecular model of the AP-1 µ1•Nef•MHC-I ternary complex (PDB ID: 4EN2) (Jia et al. 2012), rendered with the AP-2 open conformation backbone model, shown in similar orientation to A and B. Positioning of the MHC-I peptide (yellow sticks) is indicated with a white dashed oval.
RECOGNITION OF LINEAR MOTIFS BY AP-1
Studies on signal recognition by AP-1 have revealed striking similarities but also important differences in comparison with AP-2. AP-1 is a component of clathrin coats associated with the TGN and endosomes, and has been implicated in many pathways, including bidirectional transport between the TGN and endosomes (Hirst et al. 2012), export from the TGN to the plasma membrane (Guo et al. 2013), and polarized sorting from the TGN or endosomes to the basolateral domain of epithelial cells (Folsch et al. 1999; Carvajal-Gonzalez et al. 2012) and the somatodendritic domain of neurons (Dwyer et al. 2001; Margeta et al. 2009; Farias et al. 2012). AP-1 has been additionally shown to complement the role of AP-2 in endocytosis of synaptic vesicle proteins at the neuronal presynaptic terminal (Kim and Ryan 2009). The function of AP-1 in these pathways also involves recognition of linear motifs by mechanisms that are similar to those of AP-2. YXXØ and [DE]XXXL[LI] motifs bind to conserved sites on the μ1 (Ohno et al. 1995; Carvajal-Gonzalez et al. 2012; Farias et al. 2012) and γ-σ1 subunits (Janvier et al. 2003; Doray et al. 2007; Mattera et al. 2011), respectively. Moreover, the AP-1 core also occurs in equilibrium between locked (Heldwein et al. 2004) and open conformations (Fig. 3B) (Ren et al. 2013). On the other hand, the regulation of membrane recruitment and conformational activation are quite different. AP-1 binds phosphatidylinositol 4-phosphate (PtdIns(4)P), a phosphoinositide that is enriched in the TGN and endosomes, through a site on the γ subunit (Wang et al. 2003; Heldwein et al. 2004). However, this binding is too weak for efficient recruitment to membranes. Instead, the main determinant of AP-1 recruitment to membranes is binding to the GTP-bound, activated form of Arf1 (Stamnes and Rothman 1993; Traub et al. 1993). Other Arf-family members, including Arfrp1 (also known as Arl3) (Guo et al. 2013), may also play roles in AP-1 membrane recruitment. Exchange of GTP for GDP on Arf1 exposes an N-myristoylated α helix that inserts into membranes and rearranges the switch I and II regions such that they can bind AP-1. A recent study has identified two binding sites for Arf1 on the trunk domains of the γ and β1 subunits of AP-1 that contribute to recruitment of this complex to membranes (Ren et al. 2013). Strikingly, Arf1 not only promotes AP-1 membrane recruitment but also drives the conformational opening of the core that allows binding of YXXØ and [DE]XXXL[LI] motifs (Ren et al. 2013). This conformational change appears to involve pivoting on a third Arf1-binding site located on a different face of the γ trunk (Ren et al. 2013). The cooperativity of these interactions is further evidenced by the observation that both YXXØ and [DE]XXXL[LI] promote binding to each other, as well as binding of Arf1 to AP-1 (Lee et al. 2008a). The amino acids that bind Arf1 are perfectly conserved in the β2 subunit and partially conserved in the α subunit of AP-2, but the role of Arf-family members in AP-2 membrane recruitment is controversial (Krauss et al. 2003; Paleotti et al. 2005). YXXØ-, [DE]XXXL[LI]-, and Arf-binding sites are also well conserved in AP-3 (Mattera et al. 2011; Mardones et al. 2013; Ren et al. 2013), an endosome-associated adaptor involved in cargo sorting to lysosomes and lysosome-related organelles, indicating that this complex might function by a similar mechanism.
Nef: A COOPERATIVE MODIFIER OF SIGNAL–ADAPTOR INTERACTIONS
The mechanism of action of the Nef protein of human immunodeficiency virus 1 (HIV-1) exemplifies another means of regulating signal recognition by AP-1 and AP-2. Expression of Nef early in the infection cycle of HIV-1 causes down-regulation of CD4 and class I molecules of the major histocompatibility complex (MHC-I) from the surface of the host cells (i.e., T lymphocytes and macrophages) (Tokarev and Guatelli 2011). Remarkably, whereas Nef-induced CD4 down-regulation involves enhanced AP-2-dependent internalization (Jin et al. 2005; Chaudhuri et al. 2007), MHC-I down-regulation occurs by AP-1-dependent delivery of intracellular MHC-I from the TGN to endosomes (Roeth et al. 2004; Lubben et al. 2007). In both cases, the proteins are eventually transported to lysosomes for degradation. These down-regulation events involve cooperative formation of tripartite CD4•Nef•AP-2 and MHC-I•Nef•AP-1 complexes. In the CD4•Nef•AP-2 complex, a Nef carboxy-terminal flexible loop contains a [DE]XXXL[LI] motif that bind to the canonical dileucine-binding site on α-σ2 (Chaudhuri et al. 2007) and other residues that bind to a basic patch on the trunk domain of α (Chaudhuri et al. 2009). In between the dileucine and diacidic motifs there are additional hydrophobic residues that also bind to AP-2 likely via σ2 (Fig. 2B) (Jin et al. 2012). The Nef loop thus exemplifies how a long sequence containing both canonical and noncanonical elements can interact with AP-2. The dileucine motif in the CD4 tail cannot interact with the canonical dileucine-binding site on α-σ2 because the serine at position −5 from the first leucine in CD4 (Fig. 2D) is normally not phosphorylated and the dileucine-binding site on α-σ2 is occupied by the Nef loop. Instead, the dileucine motif in the CD4 tail binds to a hydrophobic patch on another region of Nef (Grzesiek et al. 1996). The cooperativity in the assembly of the tripartite complex (Chaudhuri et al. 2009) predicts the occurrence of another interaction of CD4 with AP-2 that remains to be identified. The recent resolution of the structure of the MHC-I cytosolic domain in a ternary complex with Nef and the μ1 subunit of AP-1 has revealed a different type of cooperative assembly (Jia et al. 2012). In this complex, a noncanonical tyrosine residue in the MHC-I tail occupies the binding pocket for the tyrosine residue of YXXØ signals on μ1 (Figs. 2C and 3C). The rest of the MHC-I tail binds into a groove at the Nef–μ1 interface (Jia et al. 2012). These observations raise the intriguing possibility that other cellular proteins could promote interactions of cytosolic domains with adaptors in a manner similar to Nef.
ALTERNATIVE TYROSINE-BASED SIGNAL RECOGNITION BY PTB-DOMAIN-TYPE CLASPS
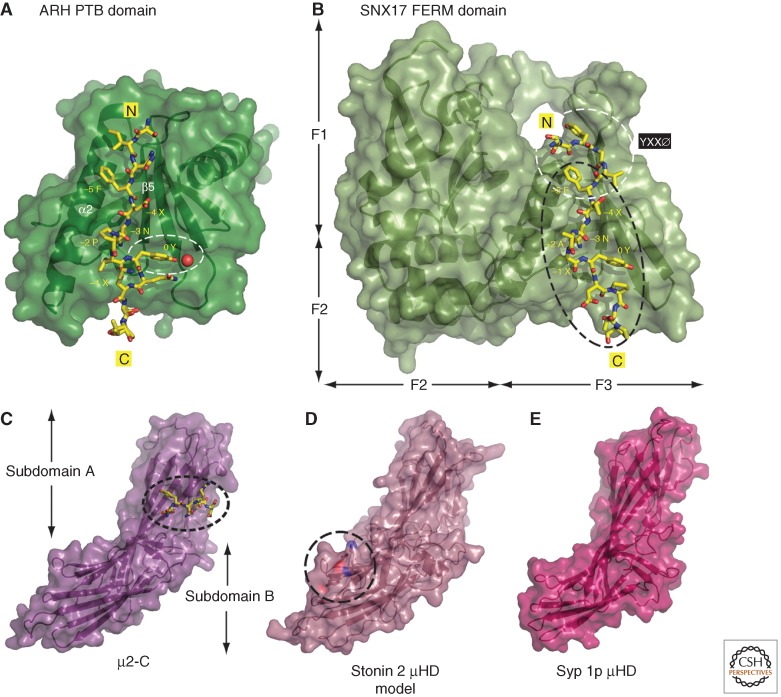
Although the [FY]XNPX[YF] signal delineated in the low-density lipoprotein (LDL) receptor (FDNPVY) first showed the importance of an anchor tyrosine to cue internalization (Davis et al. 1986), this signal is actually recognized quite differently from YXXØ signals. Substitution of the carboxy-terminal tyrosine at position 0 with phenylalanine (or even tryptophan) (Davis et al. 1986) has little effect on internalization, whereas a cysteine or alanine change diminishes uptake to that of a cytosolically truncated receptor (Davis et al. 1986; Chen et al. 1990). Several transmembrane cargoes bearing [FY]XNPX[YF] signals naturally have a phenylalanine at this position (like amnionless [Pedersen et al. 2010], Opo [Bogdanovic et al. 2012], P-selectin [Ghai et al. 2013], ROMK [Fang et al. 2009], Sanpodo [Tong et al. 2010], and TAT-1 [Nilsson et al. 2011]). Instead of AP-2, these [FY]XNPX[YF] signals are decoded by monomeric CLASPs with a phosphotyrosine-binding (PTB) domain, such as Dab2, ARH (alternatively designated LDLRAP 1), and Numb (Fig. 1) (McMahon and Boucrot 2011; Reider and Wendland 2011). The modular PTB domain in these CLASPs is similar to that originally identified in signal transduction proteins, but differs in a preference for unphosphorylated over phosphorylated tyrosine. The recent resolution of the structure of the liganded ARH PTB domain (Dvir et al. 2012) explains clearly why either tyrosine or phenylalanine can be accommodated at position 0, in stark contrast to YXXØ signals, where phenylalanine cannot replace tyrosine (Fig. 4). To engage the [YF] side chain at position 0, ARH displays a largely hydrophobic-lined acceptor cavity (Dvir et al. 2012). This is dissimilar to signal transduction PTB domains, which have an overall basic character to the larger phosphotyrosine-accommodating site (Farooq and Zhou 2004; Uhlik et al. 2005). In ARH, the tyrosine hydroxyl hydrogen bonds to a single, noncovalent water molecule to permit packing into the nonpolar surface (Fig. 4), which would be unnecessary for a phenylalanine.
Figure 4.
PTB domains and μHDs in cargo signal recognition. (A) Combined ribbon and space-filling surface representation of the ARH PTB domain bound to the LDL receptor SINFDNPVYQKT sorting-signal peptide shown in stick representation (PDB ID: 3SO6) (Dvir et al. 2012). The location of a water molecule hydrogen bonded to the position 0 tyrosine is indicated by a red sphere within the marked PTB-domain tyrosine-binding site. (B) Combined ribbon and surface representation of the SNX17 FERM-like domain complexed with the TYGVFTNAAYDPT signal from P-selection (PDB ID: 4GXB) (Ghai et al. 2013). Shown in the same relative orientation as A with the positioning of the F1–F3 subdomains indicated and the YXXØ and [FY]XNPX[YF] interaction surfaces highlighted. (C–E) Combined ribbon and surface representations of the AP-2 µ2 subunit bound to the YQRL signal from TGN38 (PDB ID:1BXX) (Owen and Evans 1998), a homology model of the human Stonin 2 mHD generated by Phyre2 (Kelley and Sternberg 2009), and the Saccharomyces cerevisiae Syp1p μHD (PDB ID: 3G9H) (Reider et al. 2009). The relative positioning of the YXXØ interaction surface in subdomain A on μ2, and the biochemically defined synaptotagmin 1 C2A-binding site on subdomain B of stonin 2 (Jung et al. 2007) are indicated. Three side chains on the stonin 2 µHD (Lys783, Tyr784, Glu785) implicated in C2A binding are highlighted.
A chemical distinction between tyrosine and phenylalanine at position 0 is that only the former can be posttranslationally phosphorylated. Because endocytic PTB domains cannot well accommodate the bulky, charged phosphotyrosine, this reversible modification can potentially govern sorting efficiency into nascent clathrin-coated structures, as with the YXXØ signal. There is some evidence for this for the LDL receptor-related protein 1 (LRP1), which binds to different partners depending on the phosphotyrosine status (Betts et al. 2008; Guttman et al. 2009). In Drosophila, the YTNPAF signal in the tetraspanin Sanpodo is recognized by Numb (Tong et al. 2010), whereas the FDNPVY signal in the scavenger receptor Draper is recognized by the PTB CLASP Ced-6 (Awasaki et al. 2006; Jha et al. 2102), and tyrosine phosphorylation regulates its uptake into cells (Fujita et al. 2012). Intriguingly, discovery-mode mass spectrometry indicates that Tyr807, the LDL receptor tyrosine 0, is phosphorylated in >125 separate studies (Hornbeck et al. 2004). Because the anchor Tyr20 of the transferrin receptor YXXØ (YTRF) signal is similarly phosphorylated in >1000 mass spectrometry analyses, focal tyrosine phosphorylation during signal relay from the cell surface might, through exclusion, underlie the formation of compositionally and/or kinetically distinct coated vesicles (Fig. 2D) (Cao et al. 1998; Mundell et al. 2006; Puthenveedu and von Zastrow 2006; Liu et al. 2010).
The structural role of all the other conserved side chains in the [FY]XNPX[YF] signal is also known. The leading −5 position tyrosine/phenylalanine packs into a complementary hydrophobic site generated principally by the so-called “selectivity” α2 helix, and usually involving a projecting PTB-domain aromatic side chain that bisects the interaction surfaces for the −5 tyrosine/phenylalanine and the −3 asparagine and −2 proline (Fig. 4). There is also additional binding energy derived from an antiparallel β augmentation, hydrogen bonding to the canonical β5 strand of the PTB domain. Yet, because these involve only main-chain contacts, the β5 strand participates rather “nonselectively” in cargo recognition (Dvir et al. 2012). At the carboxy-terminal end of the short signal β strand, the central −3 asparagine makes an intramolecular bond with tyrosine 0 to redirect the peptide main-chain configuration into a type I β turn, strongly facilitated by the following −2 proline. Overall, this structures the sorting signal to optimally engage the cognate interface of the PTB domain (Fig. 4).
MODULATION OF [FY]XNPX[YF] SIGNAL RECOGNITION BY UBIQUITINATION
Another interesting mode of modulation of the trafficking behavior of the LDL receptor FDNPVY signal is by targeted ubiquitination by an E3 ubiquitin ligase designated IDOL (for inducible degrader of the LDL receptor) (Zelcer et al. 2009). The recognition site for IDOL overlaps the FDNPVY signal; this is because substrate recognition in this particular E3 ligase stems from an atypical FERM (band 4.1/ezrin/radixin/moesin) domain that precedes the catalytic carboxy-terminal RING domain (Calkin et al. 2011; Sorrentino et al. 2011). Prior structural work on FERM domains from actin-regulating proteins like ezrin and talin revealed a tripartite fold, with the so-called F3 subdomain structurally analogous to the PTB domain (Pearson et al. 2000). The talin F3 subdomain binds to the NPXY signal in β integrins (Wegener et al. 2007), using, in part, the same basic structural arrangement as seen in ARH. Likewise, IDOL contacts physically the LDL receptor FDNPVY sorting signal, and signals in the related VLDL and ApoER2 receptors (Hong et al. 2010). The core components are also phylogenetically conserved in insects (Hong et al. 2010; Calkin et al. 2011). The IDOL ubiquitin ligase targets sites that are adjacent to the FDNPVY signal: two (Lys809) or 11 (Cys819) residues following Tyr807 (Fig. 2D) (Zelcer et al. 2009). The functional consequence of this ubiquitination is redirection of the LDL receptor to lysosomes for degradation at the expense of efficient recycling (Zelcer et al. 2009). Remarkably, initial results suggest that the IDOL-mediated polyubiquitin signal attached to the LDL receptor reroutes the protein from clathrin-dependent to clathrin-independent internalization, clearly inactivating the FDNPVY signal (Scotti et al. 2013).
OPERATION OF [FY]XNPX[YF] SIGNALS ON ENDOSOMES
Functionally analogous to YXXØ signals, [FY]XNPX[YF] signals can direct endosomal sorting events. This can happen in at least two ways: indirectly through interaction of PTB-domain-containing CLASPs with AP-1, and directly through recognition by FERM-like domain-containing sorting nexin (SNX) proteins. In one process in polarized epithelial cells, the respective adaptors, ARH and a variant of AP-1 having the μ1B subunit isoform (AP-1B), cooperate for differential sorting to the basolateral membrane from endosomes (Kang and Folsch 2011). Yet the capability of ARH to mesh with both AP-1B and AP-2 depends on the sole ARH β-subunit interaction motif (He et al. 2002; Mishra et al. 2005; Edeling et al. 2006; Schmid et al. 2006); mammalian β1 and β2 are ∼85% identical and functionally interchangeable (Keyel et al. 2008; Li et al. 2010). Consequently, the surface on the β1 appendage that ARH binds to is fully conserved in β2. ARH also has a broadly basic surface perpendicular to the [FY]XNPX[YF]-binding site that could accommodate different phosphoinositides rather than being tailored for a stereospecific isomer.
Another example of cooperation between a PTB-domain CLASP and AP-1 is provided by Numb. This protein (and the redundant paralog Numb-like) is involved in Notch signaling (Berdnik et al. 2002), cell adhesion, migration (Wang et al. 2009), and morphogenesis (Berdnik et al. 2002; Bogdanovic et al. 2012). Numb can also function on endosomes (Smith et al. 2004; Nilsson et al. 2008; McGill et al. 2009; Cotton et al. 2013; Couturier et al. 2013), and clearly has endosome-located binding partners, although biochemical interactions revolve principally around AP-2 and associated proteins (Krieger et al. 2012). The plasma membrane- and endosome-associated chordate Numb isoforms are different splice variants (Dho et al. 1999) and regulated by posttranslational phosphorylation (Smith et al. 2007; Sorensen and Conner 2008). In Drosophila, Numb cooperates with AP-1 to control Sanpodo trafficking on endosomes (Cotton et al. 2013; Couturier et al. 2013) but, in this case, basolateral recycling is prevented (Cotton et al. 2013), contrary to ARH.
A different mechanism is the recognition of [FY]XNPX[YF] signals by SNX proteins, specifically by the paralogs SNX17, SNX27, and SNX31 (Ghai et al. 2011, 2013; Bottcher et al. 2012). SNXs are a diverse class of endocytic proteins unified by the presence of a phox-homology (PX) domain selective for endosomal phosphatidylinositol 3-phosphate (PtdIns(3)P) (Cullen 2008; Teasdale and Collins 2012). SNX17, SNX27, and SNX31 stand apart from the other family members by uniquely containing an atypical FERM domain after the PX fold. The LDL receptor signal directly engages the SNX17 FERM domain (Stockinger et al. 2002; Burden et al. 2004), as do the [FY]XNPX[YF] signals in P-selectin (Florian et al. 2001; Knauth et al. 2005; Ghai et al. 2013), APP (Lee et al. 2008b), β integrins (Bottcher et al. 2012; Steinberg et al. 2012), and LRP1 (van Kerkhof et al. 2005; Donoso et al. 2009) in a phosphorylation-inhibited manner (Betts et al. 2008). The structure of the SNX17 FERM domain complexed with the P-selectin sorting signal (Ghai et al. 2013) confirms both that the F3 subdomain resembles the PTB-domain fold and that peptide binding conforms to conventional structural rules (Fig. 4). Compartmental recognition of [FY]XNPX[YF] signals is assured by the preceding PX domain, which favors endosomal PtdIns(3)P (Knauth et al. 2005; van Kerkhof et al. 2005; Ghai et al. 2011). Because of the proximity of the acceptor lysine, the IDOL-ubiquitinated FDNPVY signal in the LDL receptor may not be recognized by SNX17, explaining the diminished recycling. Strikingly, several different endocytic signals have internal or proximate lysine residues known to be ubiquitinated (Fig. 2D). This suggests that ubiquitin-dependent masking of linear sequences might be another common regulatory mechanism.
If the bulk of PTB-like domains engage [FY]XNPX[YF] signals with common structural features and a conserved binding mode, how is specificity attained? The ARH and SNX17 structures highlight another general principle for signal recognition: directly flanking residues at the amino- or carboxy-terminal end of the core sorting signal allow tailored recognition of a certain tertiary-structured binding partner. For instance, P-selectin contains a bona fide μ2-binding YXXØ-type YGVF signal interleaved with the FTNAA[YF] signal (Owen et al. 2001). In the SNX17 crystal, this peptide sequence also packs against the FERM domain F3 subdomain (Fig. 4) (Ghai et al. 2013), explaining why this could not simultaneously be recognized by AP-1 or AP-2. The β1-integrin chain similarly uses a sequence tract preceding NPKY to optimally engage SNX17 (Bottcher et al. 2012). Such extended contacts facilitate optimal conformational selection by allowing domains to discriminate between a large array of potentially similar unstructured binding peptides and select between subtly different signals. Alternatively, restricted expression patterns or temporal induction can provide specificity, as with IDOL being induced by LXR transcription factor activation (Zelcer et al. 2009).
DISTANT RELATIONS: μ-HOMOLOGY DOMAIN CLASPS
Because of the pivotal role of the μ subunit in AP-1–AP-4 cargo selection, the fact that two other protein classes deposited at clathrin assembly centers, the stonins and muniscins, also contain a μ-homology domain (μHD) (Fig. 4) is highly suggestive of further cargo recognition capabilities. The most closely related μHD is found in stonins (Andrews et al. 1996; Martina et al. 2001; Walther et al. 2001); the human stonin 2 µHD is ∼30% identical to the μ2-C domain of AP-2. In contrast, the muniscin μHD is <10% identical to μ2 accounting for the original designation FCH domain “only” (Fcho), as the initial sequence comparisons failed to identify the structural homology (Katoh 2004; Henne et al. 2007). The μHD of the yeast muniscin Syp1p is ∼14% identical to the stonin 2 domain, but neither has a similarly positioned longin-like μ-N domain, which coassembles with the β chain within the adaptor core. The stonin μHD is preceded by a phylogenetically conserved tract of ∼140 residues known as the stonin-homology domain (Martina et al. 2001) currently of unknown structure or function.
The stonins are ubiquitously expressed in chordate tissues but seem most critical in neurons; mutant alleles or silencing produces neurological phenotypes in flies, worms, and mice (Stimson et al. 2001; Estes et al. 2003; Mullen et al. 2012; Kononenko et al. 2013). At the synapse, tight exo-endocytic coupling maintains a pool of neurotransmitter-filled synaptic vesicles. Here, transmembrane cargo recognition is critical as synaptic vesicles have a highly defined protein composition (Takamori et al. 2006), and there is a remarkably conserved packaging stoichiometry for some transmembrane proteins within reforming synaptic vesicles (Takamori et al. 2006; Mutch et al. 2011). Synaptotagmin 1 is a vesicle-associated, Ca2+-responsive bilayer fusion regulator and therefore necessarily packaged into each regenerating synaptic vesicle following exocytosis. Biochemical and functional evidence indicates that the C2 domains of synaptotagmin 1 bind physically to the stonin 2 µHD (Martina et al. 2001; Walther et al. 2001; Diril et al. 2006; Jung et al. 2007). In neurons, the two proteins colocalize in puncta, and proper subcellular localization of SNT-1 depends on UNC-41 (the respective nematode orthologs) but not vice versa (Mullen et al. 2012). Because synaptotagmin (snt-1)-null worms phenocopy stonin (stn-1/unc-41) mutants (Mullen et al. 2012), a principal task of stonins is likely to promote uptake of synaptotagmin at nerve terminals.
Still, there is current controversy over precisely which presynaptic cargoes stonin 2 sorts. Worms with both snt-1 and unc-41 genes disrupted have a more severe phenotype than with either single mutation alone (Mullen et al. 2012). And forced SNT-1 overexpression does not rescue the unc-41 phenotype, unlike in Drosophila (Fergestad and Broadie 2001). Together, this suggests stonins do more than just sort synaptotagmin. A systematic CLASP RNAi screen of cultured neurons suggests that stonin 2 is actually the major adaptor for synaptic vesicle cargoes (Willox and Royle 2012), implying that it recognizes a bigger set of transmembrane proteins. But, of the synaptic vesicle constituents, only synaptotagmin 1 coimmunoprecipitates robustly with stonin 2 (Kononenko et al. 2013). And the subcellular phenotype of stonin 2-nullizygous mice disputes the notion of a general-purpose CLASP; synaptotagmin but not VAMP 2 selectively disperses over the presynaptic surface (Kononenko et al. 2013). A future challenge is to rationalize these discrepant findings.
For the muniscins, cargo partners are documented (Reider et al. 2009; Umasankar et al. 2012), but the μHDs are otherwise different in their interaction networks. This is because these μHDs are not solely engaged in cargo binding. Both the yeast and the chordate muniscin μHDs establish direct contacts with several early-arriving pioneer components of the clathrin coat (Reider et al. 2009; Henne et al. 2010; Mulkearns and Cooper 2012; Umasankar et al. 2012). The crystal structure of the Syp1p μHD leaves no doubt about the structural homology with μ2 (Fig. 4). Still, the precise interaction surface on the μHD remains to be mapped. Therefore, it is not unambiguously resolved whether cargo and endocytic proteins use the same interaction surface(s) to associate with muniscins at the plasma membrane. Certainly for the stonin 2 µHD•C2A/B interaction, both partners are folded domains, distinguishing this contact from the short peptide-based, extended conformation interactions described above.
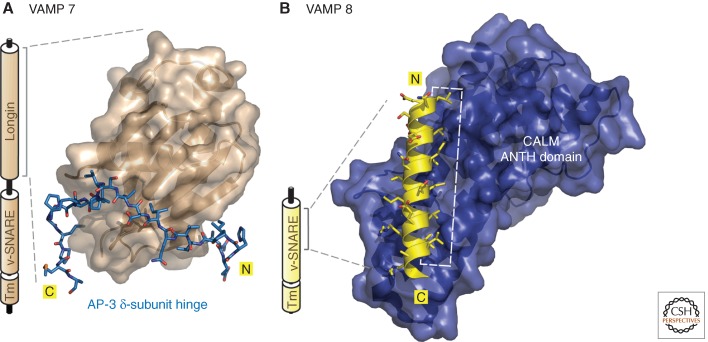
Elucidation of the molecular basis for selection of endocytic SNAREs has provided new structural insights into recognition of folded sorting signals. Because the immediate fate of donor transport vesicles is fusion with an acceptor compartment, proper packaging of SNAREs is vital. One mechanism to assure selective SNARE capture uses a disordered region of a dedicated CLASP to engage a broad molecular surface of a particular SNARE. The antithesis of the standard mode of engagement, this is how Hrb binds to VAMP 7 (Pryor et al. 2008) and, analogously, how the usually disordered hinge of the AP-3 δ subunit structures around the globular regulatory VAMP 7 longin domain to assure packaging of only a four-member cis-SNARE helical bundle (Fig. 5A) (Kent et al. 2012). The “signal” here is a radial groove along the longin-domain surface accessed by either Hrb, the δ hinge, or intramolecularly by VAMP 7 itself in the autoinhibited conformation that precludes CLASP binding. In contrast, the α-helical-solenoid fold of the CALM (and the neuronal paralog AP180) ANTH domain has evolved to selectively recognize the uncomplexed vesicle SNAREs VAMP 2, 3, and 8 (Koo et al. 2011; Miller et al. 2011). In this case, the single six-turn amphipathic α-helical tract of VAMP 8 that physically contacts the ANTH domain (Fig. 5B) also participates in SNARE helical bundling, making the two interactions mutually exclusive.
Figure 5.
Decoding folded endocytic sorting signals. (A) Schematic representation of the overall architecture of VAMP 7 with the amino-terminal longin domain, the vesicle-SNARE (v-SNARE) helical segment, and the transmembrane (Tm) domain indicated. A combined ribbon and surface representation of the globular VAMP 7 longin-domain “signal” bound by the extended AP-3 δ-subunit hinge polypeptide (blue; in stick representation) is also shown (PDB ID: 4AFI) (Kent et al. 2012). Notice the insertion of several hydrophobic side chains from the δ hinge into complementary acceptor cavities arrayed around the regulatory longin-domain circumference. (B) Schematic of the architecture of VAMP 8, which lacks the autoinhibitory longin domain. A representation of the amino-terminal portion (residues 15–38) of the α-helical v-SNARE domain of VAMP 8 complexed with the α-solenoid folded ANTH domain from CALM (PDB ID: 3ZYM) (Miller et al. 2011). The structured VAMP 8 amphipathic α helix orients seven aliphatic side chains to insert into a vertical groove along the ANTH surface (white dashed line).
CONCLUDING REMARKS AND FUTURE PERSPECTIVES
We are long past a primitive understanding of sorting signals governing coat-mediated endocytic trafficking. With elegant high-resolution structures of verified peptide sorting signals bound to their cognate interaction domains, the field has come of age. What is clear is that there is a rich variety of interaction possibilities to drive distinct sorting processes. The core primary-sequence sorting signals are four-to-six residues long, similar to the majority of eukaryotic linear motifs (ELMs) (Davey et al. 2012). Short linear sequences facilitate convergent evolution. But why is not only a single, optimized master signal present within all cargoes to be internalized from the cell surface? Certain pathogenic bacteria, for example, encode effector proteins injected into host cells, which become tyrosine phosphorylated and then bind to numerous distinct cellular SH2 domain-containing signaling regulators (Selbach et al. 2009). In general, these phosphopeptide sequences are more similar within bacteria than to the multiple endogenous SH2-binding proteins they have evolved to subvert. In fact, SH2 domains can be artificially tailored to bind phosphotyrosine signals considerably tighter (Kaneko et al. 2012). Similarly, interaction partners for clathrin-mediated endocytosis have evolved to permit relatively weak, transient but selective associations to enable efficient trafficking. It is evident then that cargo recognition folds are not optimally evolved to bind sorting signals with highest affinity. Hence, there is significant variation within and between operative signals, which have been favored to allow the flexibility and appropriate assembled dwell times necessary for normal trafficking operations. Other critical aspects of an expanded sorting-signal code are that it avoids direct competition between cargoes for uptake (Warren et al. 1998), permits discrimination between monomeric and complexed SNAREs, and allows synchronization of multiple traffic flows.
Moreover, if sorting depended solely on bimolecular cargo–coat interactions, the process would be highly error prone owing to promiscuous binding errors with related but inappropriate sequences (Ladbury and Arold 2012). And if the same signal operates at different sorting stations, it is implicit that information necessary to recruit the appropriate coat/adaptor is not the signal itself. Indeed, in AP-1 and AP-2, the cargo-recognition surfaces only become available once membrane association is established. One mechanism to increase accuracy is by temporal integration of several low-affinity contacts through spatially separate contact surfaces to guard against random noise, in the form of nonspecific univalent engagement by related (or unrelated) proteins. This robust recognition only under certain circumstances typifies selective binding of conditional peripheral membrane proteins (Moravcevic et al. 2012), and is often termed “coincidence detection” (Carlton and Cullen 2005) or a “dual-key strategy” (Itoh and De Camilli 2004). Coat assembly is therefore a probabilistic process, with increased likelihood of successful assembly with the more synchronous linked interactions at a nascent bud site. By binding to multiple adaptors or CLASPs, cargoes participate directly in the network, and thus modulate the coat assembly process (Mettlen et al. 2010). A corollary of this densely wired redundant network is that gene silencing or RNAi of numerous individual CLASPs does not have a dramatic penetrant effect on endocytosis (Garcia et al. 2001; Kang-Decker et al. 2001; Morris et al. 2002; Kamikura and Cooper 2003; Holmes et al. 2007; Koh et al. 2007; Wang et al. 2008; Chen et al. 2009; Mullen et al. 2012; Pozzi et al. 2012; Scotland et al. 2012; Suzuki et al. 2012; Umasankar et al. 2012; Kononenko et al. 2013; Tsushima et al. 2013). Rather, subtle or tissue-specific defects are manifest linked to improper signal relay by the cargo affected. This concept is also concordant with pathogenic microorganisms requiring different elements of the endocytic machinery for successful entry and infection (Eto et al. 2008; Bhattacharyya et al. 2011; Bonazzi et al. 2011; Fukumatsu et al. 2012).
It is unlikely that we currently have a complete catalog of operational sorting signals. Ongoing functional analysis reveals that within specific cargo, alterations in relative residue spacing and even tolerated loss of invariant anchor residues can occur (Kozik et al. 2010; Gephart et al. 2011; Ortega et al. 2012; Wang et al. 2012). Other noncanonical neighboring side chains compensate under these circumstances. A general screen for cytosolic sequences able to promote efficient internalization of a surface reporter highlights several novel operational sequences, yet are still heavily dependent on AP-2 (Kozik et al. 2010). First, designated “RYR,” appears initially to be an altered YXXØ-type sequence, superficially analogous to the YEQGL sequence in the P2X4 purinergic receptor (Royle et al. 2005). However, although the tyrosine in the RYR is the most vital side chain, it does not appear to bind to the YXXØ site on μ2-C subdomain A (Kozik et al. 2010). μ2 also binds to a basic stretch (devoid of aromatic residues) of the δ subunit of GABAA receptors (Gonzalez et al. 2012), so there is likely another contact surface for cargo recognition, as is evident on the μ4 subunit of AP-4 (Burgos et al. 2010). Then there are the completely novel operational sorting signals: NMDA receptors that incorporate a GluN3A subunit use a YWL sorting signal for internalization (Chowdhury et al. 2013). In this signal, the leading tyrosine can be mutated; it is not indispensable. Remarkably, although a Y971F substitution is defective, a phosphomimetic Y971E permits better AP-2 binding and internalization (Chowdhury et al. 2013). This, it seems, is a sorting signal stimulated by tyrosine phosphorylation. The signal may be related to the SWF tripeptide sequence at the end of the novel “WPK” signal, which is AP-2 and clathrin dependent (Kozik et al. 2010). In both, alanine substitution of the W[LF] pair strongly inhibits internalization (Kozik et al. 2010; Chowdhury et al. 2013). Ordered packing of unstructured regions of a CLASP over a cargo surface could also be more widespread than currently appreciated, and may allow proteins with large intrinsically disordered regions together with a folded modular domain to display cargo selectivity for more than one signal type, just like the heterotetrameric adaptors.
ACKNOWLEDGMENTS
L.M.T. is supported by the extramural program of National Institutes of Health (NIH) (R01 DK53249). J.S.B. is supported by the intramural program of NICHD, NIH. We are grateful to Bertram Canagarajah, John Guatelli, Jim Hurley, David Owen, Xuefeng Ren, and Yong Xiong for providing atomic coordinate files.
REFERENCES
*Reference is also in this collection.
- Andrews J, Smith M, Merakovsky J, Coulson M, Hannan F, Kelly LE 1996. The stoned locus of Drosophila melanogaster produces a dicistronic transcript and encodes two distinct polypeptides. Genetics 143: 1699–1711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awasaki T, Tatsumi R, Takahashi K, Arai K, Nakanishi Y, Ueda R, Ito K 2006. Essential role of the apoptotic cell engulfment genes draper and ced-6 in programmed axon pruning during Drosophila metamorphosis. Neuron 50: 855–867 [DOI] [PubMed] [Google Scholar]
- Berdnik D, Torok T, Gonzalez-Gaitan M, Knoblich J 2002. The endocytic protein α-adaptin is required for Numb-mediated asymmetric cell division in Drosophila. Dev Cell 3: 221–231 [DOI] [PubMed] [Google Scholar]
- Betts GN, van der Geer P, Komives EA 2008. Structural and functional consequences of tyrosine phosphorylation in the LRP1 cytoplasmic domain. J Biol Chem 283: 15656–15664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S, Hope TJ, Young JA 2011. Differential requirements for clathrin endocytic pathway components in cellular entry by Ebola and Marburg glycoprotein pseudovirions. Virology 419: 1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogdanovic O, Delfino-Machin M, Nicolas-Perez M, Gavilan MP, Gago-Rodrigues I, Fernandez-Minan A, Lillo C, Rios RM, Wittbrodt J, Martinez-Morales JR 2012. Numb/Numbl-Opo antagonism controls retinal epithelium morphogenesis by regulating integrin endocytosis. Dev Cell 23: 782–795 [DOI] [PubMed] [Google Scholar]
- Boll W, Ohno H, Songyang Z, Rapoport I, Cantley LC, Bonifacino JS, Kirchhausen T 1996. Sequence requirements for the recognition of tyrosine-based endocytic signals by clathrin AP-2 complexes. EMBO J 15: 5789–5795 [PMC free article] [PubMed] [Google Scholar]
- Bonazzi M, Vasudevan L, Mallet A, Sachse M, Sartori A, Prevost MC, Roberts A, Taner SB, Wilbur JD, Brodsky FM, et al. 2011. Clathrin phosphorylation is required for actin recruitment at sites of bacterial adhesion and internalization. J Cell Biol 195: 525–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonifacino JS, Traub LM 2003. Signals for sorting of transmembrane proteins to endosomes and lysosomes. Annu Rev Biochem 72: 395–447 [DOI] [PubMed] [Google Scholar]
- Bottcher RT, Stremmel C, Meves A, Meyer H, Widmaier M, Tseng HY, Fassler R 2012. Sorting nexin 17 prevents lysosomal degradation of β1 integrins by binding to the β1-integrin tail. Nat Cell Biol 14: 584–592 [DOI] [PubMed] [Google Scholar]
- Brett TJ, Traub LM, Fremont DH 2002. Accessory protein recruitment motifs in clathrin-mediated endocytosis. Structure 10: 797–809 [DOI] [PubMed] [Google Scholar]
- Brown MS, Goldstein JL 1979. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc Natl Acad Sci 76: 3330–3337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burden JJ, Sun XM, Garcia AB, Soutar AK 2004. Sorting motifs in the intracellular domain of the low density lipoprotein receptor interact with a novel domain of sorting nexin-17. J Biol Chem 279: 16237–16245 [DOI] [PubMed] [Google Scholar]
- Burgos PV, Mardones GA, Rojas AL, daSilva LL, Prabhu Y, Hurley JH, Bonifacino JS 2010. Sorting of the Alzheimer's disease amyloid precursor protein mediated by the AP-4 complex. Dev Cell 18: 425–436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calkin AC, Goult BT, Zhang L, Fairall L, Hong C, Schwabe JW, Tontonoz P 2011. FERM-dependent E3 ligase recognition is a conserved mechanism for targeted degradation of lipoprotein receptors. Proc Natl Acad Sci 108: 20107–20112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao TT, Mays RW, von Zastrow M 1998. Regulated endocytosis of G-protein-coupled receptors by a biochemically and functionally distinct subpopulation of clathrin-coated pits. J Biol Chem 273: 24592–24602 [DOI] [PubMed] [Google Scholar]
- Carlton JG, Cullen PJ 2005. Coincidence detection in phosphoinositide signaling. Trends Cell Biol 15: 540–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvajal-Gonzalez JM, Gravotta D, Mattera R, Diaz F, Bay AP, Roman AC, Schreiner RP, Thuenauer R, Bonifacino JS, Rodriguez-Boulan E 2012. Basolateral sorting of the coxsackie and adenovirus receptor through interaction of a canonical YXXPhi motif with the clathrin adaptors AP-1A and AP-1B. Proc Natl Acad Sci 109: 3820–3825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Lindwasser OW, Smith WJ, Hurley JH, Bonifacino JS 2007. Downregulation of CD4 by human immunodeficiency virus type 1 Nef is dependent on clathrin and involves direct interaction of Nef with the AP2 clathrin adaptor. J Virol 81: 3877–3890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri R, Mattera R, Lindwasser OW, Robinson MS, Bonifacino JS 2009. A basic patch on α-adaptin is required for binding of human immunodeficiency virus type 1 Nef and cooperative assembly of a CD4-Nef-AP-2 complex. J Virol 83: 2518–2530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WJ, Goldstein JL, Brown MS 1990. NPXY, a sequence often found in cytoplasmic tails, is required for coated pit-mediated internalization of the low density lipoprotein receptor. J Biol Chem 265: 3116–3123 [PubMed] [Google Scholar]
- Chen H, Ko G, Zatti A, Di Giacomo G, Liu L, Raiteri E, Perucco E, Collesi C, Min W, Zeiss C, et al. 2009. Embryonic arrest at midgestation and disruption of Notch signaling produced by the absence of both epsin 1 and epsin 2 in mice. Proc Natl Acad Sci 106: 13838–13843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury D, Marco S, Brooks IM, Zandueta A, Rao Y, Haucke V, Wesseling JF, Tavalin SJ, Perez-Otano I 2013. Tyrosine phosphorylation regulates the endocytosis and surface expression of GluN3A-containing NMDA receptors. J Neurosci 33: 4151–4164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collawn JF, Stangel M, Kuhn LA, Esekogwu V, Jing SQ, Trowbridge IS, Tainer JA 1990. Transferrin receptor internalization sequence YXRF implicates a tight turn as the structural recognition motif for endocytosis. Cell 63: 1061–1072 [DOI] [PubMed] [Google Scholar]
- Collawn JF, Kuhn LA, Liu LF, Tainer JA, Trowbridge IS 1991. Transplanted LDL and mannose-6-phosphate receptor internalization signals promote high-efficiency endocytosis of the transferrin receptor. EMBO J 10: 3247–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins BM, McCoy AJ, Kent HM, Evans PR, Owen DJ 2002. Molecular architecture and functional model of the endocytic AP2 complex. Cell 109: 523–535 [DOI] [PubMed] [Google Scholar]
- Conner SD, Schmid SL 2002. Identification of an adaptor-associated kinase, AAK1, as a regulator of clathrin-mediated endocytosis. J Cell Biol 156: 921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotton M, Benhra N, Le Borgne R 2013. Numb inhibits the recycling of Sanpodo in Drosophila sensory organ precursor. Curr Biol 23: 581–587 [DOI] [PubMed] [Google Scholar]
- Couturier L, Mazouni K, Schweisguth F 2013. Numb localizes at endosomes and controls the endosomal Sorting of Notch after asymmetric division in Drosophila. Curr Biol 23: 588–593 [DOI] [PubMed] [Google Scholar]
- Cullen PJ 2008. Endosomal sorting and signalling: An emerging role for sorting nexins. Nat Rev Mol Cell Biol 9: 574–582 [DOI] [PubMed] [Google Scholar]
- Davey NE, Van Roey K, Weatheritt RJ, Toedt G, Uyar B, Altenberg B, Budd A, Diella F, Dinkel H, Gibson TJ 2012. Attributes of short linear motifs. Mol Biosyst 8: 268–281 [DOI] [PubMed] [Google Scholar]
- Davis CG, Lehrman MA, Russell DW, Anderson RG, Brown MS, Goldstein JL 1986. The J.D. mutation in familial hypercholesterolemia: Amino acid substitution in cytoplasmic domain impedes internalization of LDL receptors. Cell 45: 15–24 [DOI] [PubMed] [Google Scholar]
- Dell'Angelica EC, Klumperman J, Stoorvogel W, Bonifacino JS 1998. Association of the AP-3 adaptor complex with clathrin. Science 280: 431–434 [DOI] [PubMed] [Google Scholar]
- Dho SE, French MB, Woods SA, McGlade CJ 1999. Characterization of four mammalian numb protein isoforms. Identification of cytoplasmic and membrane-associated variants of the phosphotyrosine binding domain. J Biol Chem 274: 33097–33104 [DOI] [PubMed] [Google Scholar]
- *.Di Fiore PP, von Zastrow M 2014. Endocytosis, signaling, and beyond. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Paolo G, De Camilli P 2006. Phosphoinositides in cell regulation and membrane dynamics. Nature 443: 651–657 [DOI] [PubMed] [Google Scholar]
- Diril MK, Wienisch M, Jung N, Klingauf J, Haucke V 2006. Stonin 2 is an AP-2-dependent endocytic sorting adaptor for synaptotagmin internalization and recycling. Dev Cell 10: 233–244 [DOI] [PubMed] [Google Scholar]
- Donoso M, Cancino J, Lee J, van Kerkhof P, Retamal C, Bu G, Gonzalez A, Caceres A, Marzolo MP 2009. Polarized traffic of LRP1 involves AP1B and SNX17 operating on Y-dependent sorting motifs in different pathways. Mol Biol Cell 20: 481–497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B, Lee I, Knisely J, Bu G, Kornfeld S 2007. The γ/σ1 and α/σ2 hemicomplexes of clathrin adaptors AP-1 and AP-2 harbor the dileucine recognition site. Mol Biol Cell 18: 1887–1896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doray B, Knisely JM, Wartman L, Bu G, Kornfeld S 2008. Identification of acidic dileucine signals in LRP9 that interact with both GGAs and AP-1/AP-2. Traffic 9: 1551–1562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drake MT, Traub LM 2001. Interaction of two structurally distinct sequence types with the clathrin terminal domain β-propeller. J Biol Chem 276: 28700–28709 [DOI] [PubMed] [Google Scholar]
- Dvir H, Shah M, Girardi E, Guo L, Farquhar MG, Zajonc DM 2012. Atomic structure of the autosomal recessive hypercholesterolemia phosphotyrosine-binding domain in complex with the LDL-receptor tail. Proc Natl Acad Sci 10: 6916–6921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer ND, Adler CE, Crump JG, L'Etoile ND, Bargmann CI 2001. Polarized dendritic transport and the AP-1 µ1 clathrin adaptor UNC-101 localize odorant receptors to olfactory cilia. Neuron 31: 277–287 [DOI] [PubMed] [Google Scholar]
- Edeling MA, Mishra SK, Keyel PA, Steinhauser AL, Collins BM, Roth R, Heuser JE, Owen DJ, Traub LM 2006. Molecular switches involving the AP-2 β2 appendage regulate endocytic cargo selection and clathrin coat assembly. Dev Cell 10: 329–342 [DOI] [PubMed] [Google Scholar]
- Estes PS, Jackson TC, Stimson DT, Sanyal S, Kelly LE, Ramaswami M 2003. Functional dissection of a eukaryotic dicistronic gene: Transgenic stonedB, but not stonedA, restores normal synaptic properties to Drosophila stoned mutants. Genetics 165: 185–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto DS, Gordon HB, Dhakal BK, Jones TA, Mulvey MA 2008. Clathrin, AP-2, and the NPXY-binding subset of alternate endocytic adaptors facilitate FimH-mediated bacterial invasion of host cells. Cell Microbiol 10: 2553–2567 [DOI] [PubMed] [Google Scholar]
- Fang L, Garuti R, Kim BY, Wade JB, Welling PA 2009. The ARH adaptor protein regulates endocytosis of the ROMK potassium secretory channel in mouse kidney. J Clin Invest 119: 3278–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farias GG, Cuitino L, Guo X, Ren X, Jarnik M, Mattera R, Bonifacino JS 2012. Signal-mediated, AP-1/clathrin-dependent sorting of transmembrane receptors to the somatodendritic domain of hippocampal neurons. Neuron 75: 810–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooq A, Zhou MM 2004. PTB or not to be: Promiscuous, tolerant and bizarro domains come of age. IUBMB Life 56: 547–557 [DOI] [PubMed] [Google Scholar]
- Fergestad T, Broadie K 2001. Interaction of stoned and synaptotagmin in synaptic vesicle endocytosis. J Neurosci 21: 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson SS, Downey WEr, Colapietro AM, Barak LS, Menard L, Caron MG 1996. Role of β-arrestin in mediating agonist-promoted G protein-coupled receptor internalization. Science 271: 363–366 [DOI] [PubMed] [Google Scholar]
- Florian V, Schluter T, Bohnensack R 2001. A new member of the sorting nexin family interacts with the C-terminus of P-selectin. Biochem Biophys Res Commun 281: 1045–1050 [DOI] [PubMed] [Google Scholar]
- Folsch H, Ohno H, Bonifacino JS, Mellman I 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99: 189–198 [DOI] [PubMed] [Google Scholar]
- Fujita Y, Nagaosa K, Shiratsuchi A, Nakanishi Y 2012. Role of NPxY motif in Draper-mediated apoptotic cell clearance in Drosophila. Drug Discov Ther 6: 291–297 [PubMed] [Google Scholar]
- Fukumatsu M, Ogawa M, Arakawa S, Suzuki M, Nakayama K, Shimizu S, Kim M, Mimuro H, Sasakawa C 2012. Shigella targets epithelial tricellular junctions and uses a noncanonical clathrin-dependent endocytic pathway to spread between cells. Cell Host Microbe 11: 325–336 [DOI] [PubMed] [Google Scholar]
- Garcia CK, Wilund K, Arca M, Zuliani G, Fellin R, Maioli M, Calandra S, Bertolini S, Cossu F, Grishin N, et al. 2001. Autosomal recessive hypercholesterolemia caused by mutations in a putative LDL receptor adaptor protein. Science 292: 1394–1398 [DOI] [PubMed] [Google Scholar]
- Gephart JD, Singh B, Higginbotham JN, Franklin JL, Gonzalez A, Folsch H, Coffey RJ 2011. Identification of a novel mono-leucine basolateral sorting motif within the cytoplasmic domain of amphiregulin. Traffic 12: 1793–1804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Mobli M, Norwood SJ, Bugarcic A, Teasdale RD, King GF, Collins BM 2011. Phox homology band 4.1/ezrin/radixin/moesin-like proteins function as molecular scaffolds that interact with cargo receptors and Ras GTPases. Proc Natl Acad Sci 108: 7763–7768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghai R, Bugarcic A, Liu H, Norwood SJ, Skeldal S, Coulson EJ, Li SS, Teasdale RD, Collins BM 2013. Structural basis for endosomal trafficking of diverse transmembrane cargos by PX-FERM proteins. Proc Natl Acad Sci 110: E643–E652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh LK, Huang F, Kim W, Gygi S, Sorkin A 2010. Multiple mechanisms collectively regulate clathrin-mediated endocytosis of the epidermal growth factor receptor. J Cell Biol 189: 871–883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez C, Moss SJ, Olsen RW 2012. Ethanol promotes clathrin adaptor-mediated endocytosis via the intracellular domain of δ-containing GABAA receptors. J Neurosci 32: 17874–17881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman OBJ, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL 1996. β-arrestin acts as a clathrin adaptor in endocytosis of the β2-adrenergic receptor. Nature 383: 447–450 [DOI] [PubMed] [Google Scholar]
- Grzesiek S, Stahl SJ, Wingfield PT, Bax A 1996. The CD4 determinant for downregulation by HIV-1 Nef directly binds to Nef. Mapping of the Nef binding surface by NMR. Biochemistry 35: 10256–10261 [DOI] [PubMed] [Google Scholar]
- Guo Y, Zanetti G, Schekman R 2013. A novel GTP-binding protein–adaptor protein complex responsible for export of Vangl2 from the trans Golgi network. Elife 2: e00160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guttman M, Betts GN, Barnes H, Ghassemian M, van der Geer P, Komives EA 2009. Interactions of the NPXY microdomains of the low density lipoprotein receptor-related protein 1. Proteomics 9: 5016–5028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Gupta S, Yi M, Michaely P, Hobbs HH, Cohen JC 2002. ARH is a modular adaptor protein that interacts with the LDL receptor, clathrin, and AP-2. J Biol Chem 277: 44044–44049 [DOI] [PubMed] [Google Scholar]
- Heldwein EE, Macia E, Wang J, Yin HL, Kirchhausen T, Harrison SC 2004. Crystal structure of the clathrin adaptor protein 1 core. Proc Natl Acad Sci 101: 14108–14113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henne WM, Kent HM, Ford MG, Hegde BG, Daumke O, Butler PJ, Mittal R, Langen R, Evans PR, McMahon HT 2007. Structure and analysis of FCHo2 F-BAR domain: A dimerizing and membrane recruitment module that effects membrane curvature. Structure 15: 839–852 [DOI] [PubMed] [Google Scholar]
- Henne WM, Boucrot E, Meinecke M, Evergren E, Vallis Y, Mittal R, McMahon HT 2010. FCHo proteins are nucleators of clathrin-mediated endocytosis. Science 328: 1281–1284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicke L, Riezman H 1996. Ubiquitination of a yeast plasma membrane receptor signals its ligand-stimulated endocytosis. Cell 84: 277–287 [DOI] [PubMed] [Google Scholar]
- Hirst J, Barlow LD, Francisco GC, Sahlender DA, Seaman MN, Dacks JB, Robinson MS 2011. The fifth adaptor protein complex. PLoS Biol 9: e1001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirst J, Borner GH, Antrobus R, Peden AA, Hodson NA, Sahlender DA, Robinson MS 2012. Distinct and overlapping roles for AP-1 and GGAs revealed by the “knocksideways” system. Curr Biol 22: 1711–1716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes A, Flett A, Coudreuse D, Korswagen HC, Pettitt J 2007. C. elegans disabled is required for cell-type specific endocytosis and is essential in animals lacking the AP-3 adaptor complex. J Cell Sci 120: 2741–2751 [DOI] [PubMed] [Google Scholar]
- Hong C, Duit S, Jalonen P, Out R, Scheer L, Sorrentino V, Boyadjian R, Rodenburg KW, Foley E, Korhonen L, et al. 2010. The E3 ubiquitin ligase IDOL induces the degradation of the low density lipoprotein receptor family members VLDLR and ApoER2. J Biol Chem 285: 19720–19726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hornbeck PV, Chabra I, Kornhauser JM, Skrzypek E, Zhang B 2004. PhosphoSite: A bioinformatics resource dedicated to physiological protein phosphorylation. Proteomics 4: 1551–1561 [DOI] [PubMed] [Google Scholar]
- Howard JP, Hutton JL, Olson JM, Payne GS 2002. Sla1p serves as the targeting signal recognition factor for NPFX(1,2)D-mediated endocytosis. J Cell Biol 157: 315–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang F, Jiang X, Sorkin A 2003. Tyrosine phosphorylation of the β2 subunit of clathrin adaptor complex AP-2 reveals the role of a di-leucine motif in the epidermal growth factor receptor trafficking. J Biol Chem 278: 43411–43417 [DOI] [PubMed] [Google Scholar]
- Itoh T, De Camilli P 2004. Membrane trafficking: Dual-key strategy. Nature 429: 141–143 [DOI] [PubMed] [Google Scholar]
- Jackson LP, Kelly BT, McCoy AJ, Gaffry T, James LC, Collins BM, Honing S, Evans PR, Owen DJ 2010. A large-scale conformational change couples membrane recruitment to cargo binding in the AP2 clathrin adaptor complex. Cell 141: 1220–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadot M, Canfield WM, Gregory W, Kornfeld S 1992. Characterization of the signal for rapid internalization of the bovine mannose 6-phosphate/insulin-like growth factor-II receptor. J Biol Chem 267: 11069–11077 [PubMed] [Google Scholar]
- Janvier K, Kato Y, Boehm M, Rose JR, Martina JA, Kim BY, Venkatesan S, Bonifacino JS 2003. Recognition of dileucine-based sorting signals from HIV-1 Nef and LIMP-II by the AP-1 γ-σ1 and AP-3 δ-σ3 hemicomplexes. J Cell Biol 163: 1281–1290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A, Watkins SC, Traub LM 2102. The apoptotic engulfment protein Ced-6 participates in clathrin-mediated yolk uptake in Drosophila egg chambers. Mol Biol Cell 23: 1742–1764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X, Singh R, Homann S, Yang H, Guatelli J, Xiong Y 2012. Structural basis of evasion of cellular adaptive immunity by HIV-1 Nef. Nat Struct Mol Biol 19: 701–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YJ, Cai CY, Zhang X, Zhang HT, Hirst JA, Burakoff SJ 2005. HIV Nef-mediated CD4 down-regulation is adaptor protein complex 2 dependent. J Immunol 175: 3157–3164 [DOI] [PubMed] [Google Scholar]
- Jin YJ, Cai CY, Mezei M, Ohlmeyer M, Sanchez R, Burakoff SJ 2012. Identification of a novel binding site between HIV Type 1 Nef C-terminal flexible loop and AP2 required for Nef-mediated CD4 downregulation. AIDS Res Hum Retroviruses 29: 725–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KF, Kornfeld S 1992. The cytoplasmic tail of the mannose 6-phosphate/insulin-like growth factor-II receptor has two signals for lysosomal enzyme sorting in the Golgi. J Cell Biol 119: 249–257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung N, Wienisch M, Gu M, Rand JB, Muller SL, Krause G, Jorgensen EM, Klingauf J, Haucke V 2007. Molecular basis of synaptic vesicle cargo recognition by the endocytic sorting adaptor stonin 2. J Cell Biol 179: 1497–1510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamikura DM, Cooper JA 2003. Lipoprotein receptors and a disabled family cytoplasmic adaptor protein regulate EGL-17/FGF export in C. elegans. Genes Dev 17: 2798–2811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko T, Huang H, Cao X, Li X, Li C, Voss C, Sidhu SS, Li SS 2012. Superbinder SH2 domains act as antagonists of cell signaling. Sci Signal 5: ra68. [DOI] [PubMed] [Google Scholar]
- Kang RS, Folsch H 2011. ARH cooperates with AP-1B in the exocytosis of LDLR in polarized epithelial cells. J Cell Biol 193: 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Decker N, Mantchev GT, Juneja SC, McNiven MA, van Deursen JM 2001. Lack of acrosome formation in Hrb-deficient mice. Science 294: 1531–1533 [DOI] [PubMed] [Google Scholar]
- Katoh M 2004. Identification and characterization of human FCHO2 and mouse Fcho2 genes in silico. Int J Mol Med 14: 327–331 [PubMed] [Google Scholar]
- Kelley LA, Sternberg MJ 2009. Protein structure prediction on the Web: A case study using the Phyre server. Nat Protoc 4: 363–371 [DOI] [PubMed] [Google Scholar]
- Kelly BT, Owen DJ 2011. Endocytic sorting of transmembrane protein cargo. Curr Opin Cell Biol 23: 404–412 [DOI] [PubMed] [Google Scholar]
- Kelly BT, McCoy AJ, Spate K, Miller SE, Evans PR, Honing S, Owen DJ 2008. A structural explanation for the binding of endocytic dileucine motifs by the AP2 complex. Nature 456: 976–979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kent HM, Evans PR, Schafer IB, Gray SR, Sanderson CM, Luzio JP, Peden AA, Owen DJ 2012. Structural basis of the intracellular sorting of the SNARE VAMP7 by the AP3 adaptor complex. Dev Cell 22: 979–988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keyel PA, Thieman JR, Roth R, Erkan E, Everett ET, Watkins SC, Traub LM 2008. The AP-2 adaptor b2 appendage scaffolds alternate cargo endocytosis. Mol Biol Cell 19: 5309–5326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Ryan TA 2009. Synaptic vesicle recycling at CNS synapses without AP-2. J Neurosci 29: 3865–3874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Kirchhausen T 2014. Clathrin-mediated endocytosis. I: Structure and design of CCVs. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knauth P, Schluter T, Czubayko M, Kirsch C, Florian V, Schreckenberger S, Hahn H, Bohnensack R 2005. Functions of sorting nexin 17 domains and recognition motif for P-selectin trafficking. J Mol Biol 347: 813–825 [DOI] [PubMed] [Google Scholar]
- Koh TW, Korolchuk VI, Wairkar YP, Jiao W, Evergren E, Pan H, Zhou Y, Venken KJ, Shupliakov O, Robinson IM, et al. 2007. Eps15 and Dap160 control synaptic vesicle membrane retrieval and synapse development. J Cell Biol 178: 309–322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kononenko NL, Diril MK, Puchkov D, Kintscher M, Koo SJ, Pfuhl G, Winter Y, Wienisch M, Klingauf J, Breustedt J, et al. 2013. Compromised fidelity of endocytic synaptic vesicle protein sorting in the absence of stonin 2. Proc Natl Acad Sci 110: E526–E535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo SJ, Markovic S, Puchkov D, Mahrenholz CC, Beceren-Braun F, Maritzen T, Dernedde J, Volkmer R, Oschkinat H, Haucke V 2011. SNARE motif-mediated sorting of synaptobrevin by the endocytic adaptors clathrin assembly lymphoid myeloid leukemia (CALM) and AP180 at synapses. Proc Natl Acad Sci 108: 13540–13545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozik P, Francis RW, Seaman MN, Robinson MS 2010. A screen for endocytic motifs. Traffic 11: 843–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss M, Kinuta M, Wenk MR, De Camilli P, Takei K, Haucke V 2003. ARF6 stimulates clathrin/AP-2 recruitment to synaptic membranes by activating phosphatidylinositol phosphate kinase type Iγ. J Cell Biol 162: 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieger JR, Taylor P, Gajadhar AS, Guha A, Moran MF, McGlade CJ 2012. Identification and SRM quantification of endocytosis factors associated with Numb. Mol Cell Proteomics 12: 499–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladbury JE, Arold ST 2012. Noise in cellular signaling pathways: Causes and effects. Trends Biochem Sci 37: 173–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Doray B, Govero J, Kornfeld S 2008a. Binding of cargo sorting signals to AP-1 enhances its association with ADP ribosylation factor 1-GTP. J Cell Biol 180: 467–472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Retamal C, Cuitino L, Caruano-Yzermans A, Shin JE, van Kerkhof P, Marzolo MP, Bu G 2008b. Adaptor protein sorting nexin 17 regulates amyloid precursor protein trafficking and processing in the early endosomes. J Biol Chem 283: 11501–11508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letourneur F, Klausner RD 1992. A novel di-leucine motif and a tyrosine-based motif independently mediate lysosomal targeting and endocytosis of CD3 chains. Cell 69: 1143–1157 [DOI] [PubMed] [Google Scholar]
- Li W, Puertollano-Moro R, Bonifacino J, Overbeek P, Everett E 2010. Disruption of the murine Ap2β1 gene causes nonsyndromic cleft palate. Cleft Palate Craniofac J 47: 566–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindwasser OW, Smith WJ, Chaudhuri R, Yang P, Hurley JH, Bonifacino JS 2008. A diacidic motif in human immunodeficiency virus type 1 Nef is a novel determinant of binding to AP-2. J Virol 82: 1166–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu AP, Aguet F, Danuser G, Schmid SL 2010. Local clustering of transferrin receptors promotes clathrin-coated pit initiation. J Cell Biol 191: 1381–1393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lubben NB, Sahlender DA, Motley AM, Lehner PJ, Benaroch P, Robinson MS 2007. HIV-1 Nef-induced down-regulation of MHC class I requires AP-1 and clathrin but not PACS-1 and is impeded by AP-2. Mol Biol Cell 18: 3351–3365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mardones GA, Burgos PV, Lin Y, Kloer DP, Magadan JG, Hurley JH, Bonifacino JS 2013. Structural basis for the recognition of tyrosine-based sorting signals by the μ3A subunit of the AP-3 adaptor complex. J Biol Chem 288: 9563–9571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeta MA, Wang GJ, Shen K 2009. Clathrin adaptor AP-1 complex excludes multiple postsynaptic receptors from axons in C. elegans. Proc Natl Acad Sci 106: 1632–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martina JA, Bonangelino CJ, Aguilar RC, Bonifacino JS 2001. Stonin 2: An adaptor-like protein that interacts with components of the endocytic machinery. J Cell Biol 153: 1111–1120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Boehm M, Chaudhuri R, Prabhu Y, Bonifacino JS 2011. Conservation and diversification of dileucine signal recognition by adaptor protein (AP) complex variants. J Biol Chem 286: 2022–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGill MA, Dho SE, Weinmaster G, McGlade CJ 2009. Numb regulates post-endocytic trafficking and degradation of Notch1. J Biol Chem 284: 26427–26438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon HT, Boucrot E 2011. Molecular mechanism and physiological functions of clathrin-mediated endocytosis. Nat Rev Mol Cell Biol 12: 517–533 [DOI] [PubMed] [Google Scholar]
- Mettlen M, Loerke D, Yarar D, Danuser G, Schmid SL 2010. Cargo- and adaptor-specific mechanisms regulate clathrin-mediated endocytosis. J Cell Biol 188: 919–933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SE, Sahlender DA, Graham SC, Honing S, Robinson MS, Peden AA, Owen DJ 2011. The molecular basis for the endocytosis of small R-SNAREs by the clathrin adaptor CALM. Cell 147: 1118–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Hawryluk MJ, Agostinelli NR, Watkins SC, Traub LM 2002. Disabled-2 exhibits the properties of a cargo-selective endocytic clathrin adaptor. EMBO J 21: 4915–4926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra SK, Keyel PA, Edeling MA, Owen DJ, Traub LM 2005. Functional dissection of an AP-2 β2 appendage-binding sequence within the autosomal recessive hypercholesterolemia (ARH) protein. J Biol Chem 280: 19270–19280 [DOI] [PubMed] [Google Scholar]
- Moravcevic K, Oxley CL, Lemmon MA 2012. Conditional peripheral membrane proteins: Facing up to limited specificity. Structure 20: 15–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris SM, Tallquist MD, Rock CO, Cooper JA 2002. Dual roles for the Dab2 adaptor protein in embryonic development and kidney transport. EMBO J 21: 1555–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulkearns EE, Cooper JA 2012. FCHO2 organizes clathrin-coated structures and interacts with Dab2 for LDLR endocytosis. Mol Biol Cell 23: 1330–1342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen GP, Grundahl KM, Gu M, Watanabe S, Hobson RJ, Crowell JA, McManus JR, Mathews EA, Jorgensen EM, Rand JB 2012. UNC-41/Stonin functions with AP2 to recycle synaptic vesicles in Caenorhabditis elegans. PLoS ONE 7: e40095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mundell SJ, Luo J, Benovic JL, Conley PB, Poole AW 2006. Distinct clathrin-coated pits sort different G protein-coupled receptor cargo. Traffic 7: 1420–1431 [DOI] [PubMed] [Google Scholar]
- Mutch SA, Kensel-Hammes P, Gadd JC, Fujimoto BS, Allen RW, Schiro PG, Lorenz RM, Kuyper CL, Kuo JS, Bajjalieh SM, et al. 2011. Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J Neurosci 31: 1461–1470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Conradt B, Ruaud AF, Chen CC, Hatzold J, Bessereau JL, Grant BD, Tuck S 2008. Caenorhabditis elegans num-1 negatively regulates endocytic recycling. Genetics 179: 375–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L, Jonsson E, Tuck S 2011. Caenorhabditis elegans numb inhibits endocytic recycling by binding TAT-1 aminophospholipid translocase. Traffic 12: 1839–1849 [DOI] [PubMed] [Google Scholar]
- Ohno H, Stewart J, Fournier MC, Bosshart H, Rhee I, Miyatake S, Saito T, Gallusser A, Kirchhausen T, Bonifacino JS 1995. Interaction of tyrosine-based sorting signals with clathrin-associated proteins. Science 269: 1872–1875 [DOI] [PubMed] [Google Scholar]
- Ohno H, Fournier MC, Poy G, Bonifacino JS 1996. Structural determinants of interaction of tyrosine-based sorting signals with the adaptor medium chains. J Biol Chem 271: 29009–29015 [DOI] [PubMed] [Google Scholar]
- Ohno H, Aguilar RC, Yeh D, Taura D, Saito T, Bonifacino JS 1998. The medium subunits of adaptor complexes recognize distinct but overlapping sets of tyrosine-based sorting signals. J Biol Chem 273: 25915–25921 [DOI] [PubMed] [Google Scholar]
- Olusanya O, Andrews PD, Swedlow JR, Smythe E 2001. Phosphorylation of threonine 156 of the μ2 subunit of the AP2 complex is essential for endocytosis in vitro and in vivo. Curr Biol 11: 896–900 [DOI] [PubMed] [Google Scholar]
- Ortega B, Mason AK, Welling PA 2012. A tandem di-hydrophobic motif mediates clathrin-dependent endocytosis via direct binding to the AP-2 ασ2 subunits. J Biol Chem 287: 26867–26875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Evans PR 1998. A structural explanation for the recognition of tyrosine-based endocytotic signals. Science 282: 1327–1332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen DJ, Setiadi H, Evans PR, McEver RP, Green SA 2001. A third specificity-determining site in μ2 adaptin for sequences upstream of YxxΦ sorting motifs. Traffic 2: 105–110 [DOI] [PubMed] [Google Scholar]
- Paleotti O, Macia E, Luton F, Klein S, Partisani M, Chardin P, Kirchhausen T, Franco M 2005. The small G-protein Arf6GTP recruits the AP-2 adaptor complex to membranes. J Biol Chem 280: 21661–21666 [DOI] [PubMed] [Google Scholar]
- Pearson MA, Reczek D, Bretscher A, Karplus PA 2000. Structure of the ERM protein moesin reveals the FERM domain fold masked by an extended actin binding tail domain. Cell 101: 259–270 [DOI] [PubMed] [Google Scholar]
- Pedersen GA, Chakraborty S, Steinhauser AL, Traub LM, Madsen M 2010. AMN directs endocytosis of the intrinsic factor-vitamin B12 receptor cubam by engaging ARH or Dab2. Traffic 11: 706–720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Piper RC, Dikic I, Lukacs G 2014. Ubiquitin-dependent sorting in endocytosis. Cold Spring Harb Perspect Biol 10.1101/cshperspect.a016808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitcher C, Honing S, Fingerhut A, Bowers K, Marsh M 1999. Cluster of differentiation antigen 4 (CD4) endocytosis and adaptor complex binding require activation of the CD4 endocytosis signal by serine phosphorylation. Mol Biol Cell 10: 677–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polo S, Sigismund S, Faretta M, Guidi M, Capua MR, Bossi G, Chen H, De Camilli P, Di Fiore PP 2002. A single motif responsible for ubiquitin recognition and monoubiquitination in endocytic proteins. Nature 416: 451–455 [DOI] [PubMed] [Google Scholar]
- Pond L, Kuhn LA, Teyton L, Schutze MP, Tainer JA, Jackson MR, Peterson PA 1995. A role for acidic residues in di-leucine motif-based targeting to the endocytic pathway. J Biol Chem 270: 19989–19997 [DOI] [PubMed] [Google Scholar]
- Pozzi B, Amodio S, Lucano C, Sciullo A, Ronzoni S, Castelletti D, Adler T, Treise I, Betsholtz IH, Rathkolb B, et al. 2012. The endocytic adaptor eps15 controls marginal zone B cell numbers. PLoS ONE 7: e50818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhu Y, Burgos PV, Schindler C, Farias GG, Magadan JG, Bonifacino JS 2012. Adaptor protein 2-mediated endocytosis of the β-secretase BACE1 is dispensable for amyloid precursor protein processing. Mol Biol Cell 23: 2339–2351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pryor PR, Jackson L, Gray SR, Edeling MA, Thompson A, Sanderson CM, Evans PR, Owen DJ, Luzio JP 2008. Molecular basis for the sorting of the SNARE VAMP7 into endocytic clathrin-coated vesicles by the ArfGAP Hrb. Cell 134: 817–827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puthenveedu MA, von Zastrow M 2006. Cargo regulates clathrin-coated pit dynamics. Cell 127: 113–124 [DOI] [PubMed] [Google Scholar]
- Reider A, Wendland B 2011. Endocytic adaptors—Social networking at the plasma membrane. J Cell Sci 124: 1613–1622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reider A, Barker SL, Mishra SK, Im YJ, Maldonado-Baez L, Hurley JH, Traub LM, Wendland B 2009. Syp1 is a conserved endocytic adaptor that contains domains involved in cargo selection and membrane tubulation. EMBO J 28: 3103–3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren X, Farias GG, Canagarajah BJ, Bonifacino JS, Hurley JH 2013. Structural basis for recruitment and activation of the AP-1 clathrin adaptor complex by Arf1. Cell 152: 755–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricotta D, Conner SD, Schmid SL, von Figura K, Honing S 2002. Phosphorylation of the AP2 mu subunit by AAK1 mediates high affinity binding to membrane protein sorting signals. J Cell Biol 156: 791–795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson MS 2004. Adaptable adaptors for coated vesicles. Trends Cell Biol 14: 167–174 [DOI] [PubMed] [Google Scholar]
- Roeth JF, Williams M, Kasper MR, Filzen TM, Collins KL 2004. HIV-1 Nef disrupts MHC-I trafficking by recruiting AP-1 to the MHC-I cytoplasmic tail. J Cell Biol 167: 903–913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer J, Schweizer A, Russell D, Kornfeld S 1996. The targeting of Lamp1 to lysosomes is dependent on the spacing of its cytoplasmic tail tyrosine sorting motif relative to the membrane. J Cell Biol 132: 565–576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle SJ, Qureshi OS, Bobanovic LK, Evans PR, Owen DJ, Murrell-Lagnado RD 2005. Non-canonical YXXGF endocytic motifs: Recognition by AP2 and preferential utilization in P2X4 receptors. J Cell Sci 118: 3073–3080 [DOI] [PubMed] [Google Scholar]
- Schmid EM, Ford MG, Burtey A, Praefcke GJ, Peak Chew SY, Mills IG, Benmerah A, McMahon HT 2006. Role of the AP2 β-appendage hub in recruiting partners for clathrin coated vesicle assembly. PLoS Biol 4: e262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schweizer A, Kornfeld S, Rohrer J 1996. Cysteine34 of the cytoplasmic tail of the cation-dependent mannose 6-phosphate receptor is reversibly palmitoylated and required for normal trafficking and lysosomal enzyme sorting. J Cell Biol 132: 577–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland PB, Heath JL, Conway AE, Porter NB, Armstrong MB, Walker JA, Klebig ML, Lavau CP, Wechsler DS 2012. The PICALM protein plays a key role in iron homeostasis and cell proliferation. PLoS ONE 7: e44252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotti E, Calamai M, Goulbourne CN, Zhang L, Hong C, Lin RR, Choi J, Pilch PF, Fong LG, Zou P, et al. 2013. IDOL stimulates clathrin-independent endocytosis and MVB-mediated lysosomal degradation of the LDLR. Mol Cell Biol 33: 1503–1514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selbach M, Paul FE, Brandt S, Guye P, Daumke O, Backert S, Dehio C, Mann M 2009. Host cell interactome of tyrosine-phosphorylated bacterial proteins. Cell Host Microbe 5: 397–403 [DOI] [PubMed] [Google Scholar]
- Shih SC, Katzmann DJ, Schnell JD, Sutanto M, Emr SD, Hicke L 2002. Epsins and Vps27p/Hrs contain ubiquitin-binding domains that function in receptor endocytosis. Nat Cell Biol 4: 389–393 [DOI] [PubMed] [Google Scholar]
- Shiratori T, Miyatake S, Ohno H, Nakaseko C, Isono K, Bonifacino JS, Saito T 1997. Tyrosine phosphorylation controls internalization of CTLA-4 by regulating its interaction with clathrin-associated adaptor complex AP-2. Immunity 6: 583–589 [DOI] [PubMed] [Google Scholar]
- Smith CA, Dho SE, Donaldson J, Tepass U, McGlade CJ 2004. The cell fate determinant numb interacts with EHD/Rme-1 family proteins and has a role in endocytic recycling. Mol Biol Cell 15: 3698–3708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CA, Lau KM, Rahmani Z, Dho SE, Brothers G, She YM, Berry DM, Bonneil E, Thibault P, Schweisguth F, et al. 2007. aPKC-mediated phosphorylation regulates asymmetric membrane localization of the cell fate determinant Numb. EMBO J 26: 468–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorensen EB, Conner SD 2008. AAK1 regulates numb function at an early step in clathrin-mediated endocytosis. Traffic 9: 1791–1800 [DOI] [PubMed] [Google Scholar]
- Sorrentino V, Scheer L, Santos A, Reits E, Bleijlevens B, Zelcer N 2011. Distinct functional domains contribute to degradation of the low density lipoprotein receptor (LDLR) by the E3 ubiquitin ligase inducible Degrader of the LDLR (IDOL). J Biol Chem 286: 30190–30199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamnes MA, Rothman JE 1993. The binding of AP-1 clathrin adaptor particles to Golgi membranes requires ADP-ribosylation factor, a small GTP-binding protein. Cell 73: 999–1005 [DOI] [PubMed] [Google Scholar]
- Steinberg F, Heesom KJ, Bass MD, Cullen PJ 2012. SNX17 protects integrins from degradation by sorting between lysosomal and recycling pathways. J Cell Biol 197: 219–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stimson DT, Estes PS, Rao S, Krishnan KS, Kelly LE, Ramaswami M 2001. Drosophila stoned proteins regulate the rate and fidelity of synaptic vesicle internalization. J Neurosci 21: 3034–3044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockinger W, Sailler B, Strasser V, Recheis B, Fasching D, Kahr L, Schneider WJ, Nimpf J 2002. The PX-domain protein SNX17 interacts with members of the LDL receptor family and modulates endocytosis of the LDL receptor. EMBO J 21: 4259–4267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Tanaka H, Tanimura A, Tanabe K, Oe N, Rai S, Kon S, Fukumoto M, Takei K, Abe T, et al. 2012. The clathrin assembly protein PICALM is required for erythroid maturation and transferrin internalization in mice. PLoS ONE 7: e31854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takamori S, Holt M, Stenius K, Lemke EA, Gronborg M, Riedel D, Urlaub H, Schenck S, Brugger B, Ringler P, et al. 2006. Molecular anatomy of a trafficking organelle. Cell 127: 831–846 [DOI] [PubMed] [Google Scholar]
- Tan PK, Howard JP, Payne GS 1996. The sequence NPFXD defines a new class of endocytosis signal in Saccharomyces cerevisiae. J Cell Biol 135: 1789–1800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teasdale RD, Collins BM 2012. Insights into the PX (phox-homology) domain and SNX (sorting nexin) protein families: Structures, functions and roles in disease. Biochem J 441: 39–59 [DOI] [PubMed] [Google Scholar]
- Tokarev A, Guatelli J 2011. Misdirection of membrane trafficking by HIV-1 Vpu and Nef: Keys to viral virulence and persistence. Cell Logist 1: 90–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong X, Zitserman D, Serebriiskii I, Andrake M, Dunbrack R, Roegiers F 2010. Numb independently antagonizes Sanpodo membrane targeting and Notch signaling in Drosophila sensory organ precursor cells. Mol Biol Cell 21: 802–810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub LM 2009. Tickets to ride: Selecting cargo for clathrin-regulated internalization. Nat Rev Mol Cell Biol 10: 583–596 [DOI] [PubMed] [Google Scholar]
- Traub LM, Ostrom JA, Kornfeld S 1993. Biochemical dissection of AP-1 recruitment onto Golgi membranes. J Cell Biol 123: 561–573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsushima H, Malabarba MG, Confalonieri S, Senic-Matuglia F, Verhoef LG, Bartocci C, D'Ario G, Cocito A, Di Fiore PP, Salcini AE 2013. A snapshot of the physical and functional wiring of the eps15 homology domain network in the nematode. PLoS ONE 8: e56383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlik MT, Temple B, Bencharit S, Kimple AJ, Siderovski DP, Johnson GL 2005. Structural and evolutionary division of phosphotyrosine binding (PTB) domains. J Mol Biol 345: 1–20 [DOI] [PubMed] [Google Scholar]
- Umasankar PK, Sanker S, Thieman JR, Chakraborty S, Wendland B, Tsang M, Traub LM 2012. Distinct and separable activities of the endocytic clathrin-coat components Fcho1/2 and AP-2 in developmental patterning. Nat Cell Biol 14: 488–501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kerkhof P, Lee J, McCormick L, Tetrault E, Lu W, Schoenfish M, Oorschot V, Strous GJ, Klumperman J, Bu G 2005. Sorting nexin 17 facilitates LRP recycling in the early endosome. EMBO J 24: 2851–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voorhees P, Deignan E, van Donselaar E, Humphrey J, Marks MS, Peters PJ, Bonifacino JS 1995. An acidic sequence within the cytoplasmic domain of furin functions as a determinant of trans-Golgi network localization and internalization from the cell surface. EMBO J 14: 4961–4975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther K, Krauss M, Diril MK, Lemke S, Ricotta D, Honing S, Kaiser S, Haucke V 2001. Human stoned B interacts with AP-2 and synaptotagmin and facilitates clathrin-coated vesicle uncoating. EMBO Rep 2: 634–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YJ, Wang J, Sun HQ, Martinez M, Sun YX, Macia E, Kirchhausen T, Albanesi JP, Roth MG, Yin HL 2003. Phosphatidylinositol 4 phosphate regulates targeting of clathrin adaptor AP-1 complexes to the Golgi. Cell 114: 299–310 [DOI] [PubMed] [Google Scholar]
- Wang W, Bouhours M, Gracheva EO, Liao EH, Xu K, Sengar AS, Xin X, Roder J, Boone C, Richmond JE, et al. 2008. ITSN-1 controls vesicle recycling at the neuromuscular junction and functions in parallel with DAB-1. Traffic 9: 742–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Sandiford S, Wu C, Li SS 2009. Numb regulates cell-cell adhesion and polarity in response to tyrosine kinase signalling. EMBO J 28: 2360–2373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Sato K, Otsuka Y, Otsu W, Inaba M 2012. Clathrin-mediated endocytosis of mammalian erythroid AE1 anion exchanger facilitated by a YXXΦ or a noncanonical YXXXΦ motif in the N-terminal stretch. J Vet Med Sci 74: 17–25 [DOI] [PubMed] [Google Scholar]
- Warren RA, Green FA, Stenberg PE, Enns CA 1998. Distinct saturable pathways for the endocytosis of different tyrosine motifs. J Biol Chem 273: 17056–17063 [DOI] [PubMed] [Google Scholar]
- Wegener KL, Partridge AW, Han J, Pickford AR, Liddington RC, Ginsberg MH, Campbell ID 2007. Structural basis of integrin activation by talin. Cell 128: 171–182 [DOI] [PubMed] [Google Scholar]
- Willox AK, Royle SJ 2012. Stonin 2 is a major adaptor protein for clathrin-mediated synaptic vesicle retrieval. Curr Biol 22: 1435–1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A, Xing Y, Harrison SC, Kirchhausen T 2010. Structural analysis of the interaction between Dishevelled2 and clathrin AP-2 adaptor, a critical step in noncanonical Wnt signaling. Structure 18: 1311–1320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelcer N, Hong C, Boyadjian R, Tontonoz P 2009. LXR regulates cholesterol uptake through Idol-dependent ubiquitination of the LDL receptor. Science 325: 100–104 [DOI] [PMC free article] [PubMed] [Google Scholar]