Figure 5.
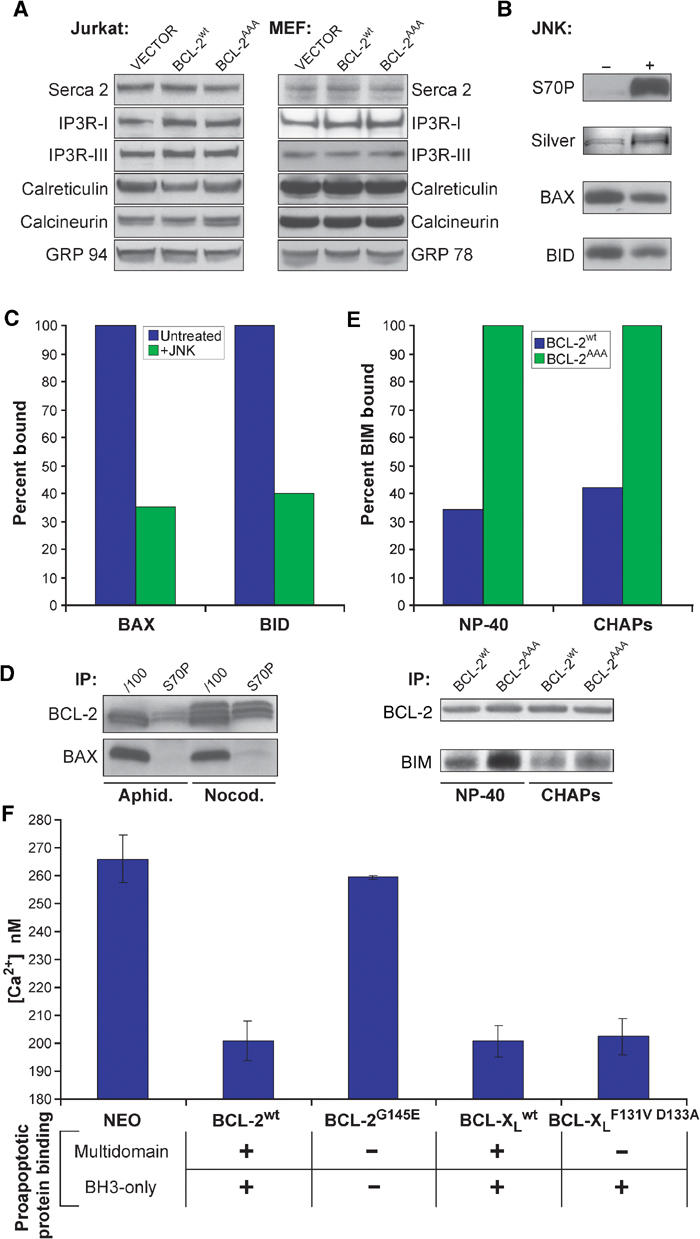
BCL-2 must bind to proapoptotic proteins to lower ER calcium, and this binding is prevented by phosphorylation. (A) RIPA extracts of Jurkat cell lines were analyzed for levels of calcium handling proteins. (B) GST-BCL-2 was treated with JNK kinase, run on SDS–PAGE, and either silver stained or Western blotted with anti-S70P antibody. (C) This protein was then mixed with purified BAX or BID protein in NP-40 buffer, captured with GSH beads, and association with BAX or BID was assessed by Western blot and quantitated by densitometry. (D) Jurkat cells were treated with aphidicolin or nocodazole for 16 h, and then whole-cell extracts were made in IP buffer, immunoprecipitated with the indicated antibody, and then assayed by Western blot. (E) Following subcellular fractionation, light membrane fractions from MEF lines were solubilized in NP-40 or CHAPS IP buffer, immunoprecipitated with anti-BCL-2 antibody, and assayed for associated BIM by Western blot. (F) Fl 5.12 lines expressing the indicated constructs were loaded with Fura-2, and peak calcium following thapsigargin treatment was measured.
