Figure 2.
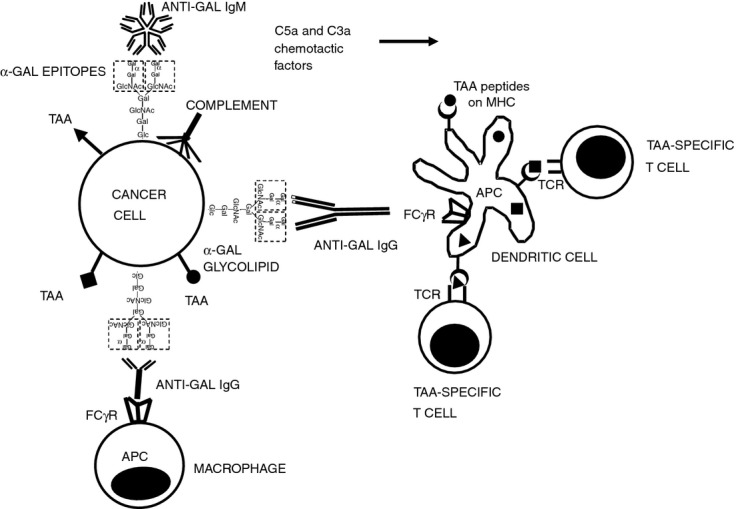
Targeting of tumour cells to antigen-presenting cell (APC) by the natural anti-Gal antibody. Following insertion of injected α-gal glycolipids into tumour cell membranes both anti-Gal IgM and IgG antibodies (released from ruptured capillaries) bind to α-gal epitopes on the tumour cells. Binding of anti-Gal IgM results in effective complement activation and generation of the chemotactic complement cleavage peptides C5a and C3a, which induce rapid migration of APC (dendritic cells and macrophages) into the treated tumour. Following anti-Gal IgG binding to the α-gal epitopes on tumour cells (i.e. opsonization), its Fc portion interacts with Fcγ receptor (FcγR) on APC. This interaction stimulates APC to internalize intact or lysed tumour cells and the tumour-associated antigens (TAA) they carry. Internalized TAA are transported by the APC to regional lymph nodes, processed and various immunogenic TAA peptides (•, ▪, ▴) are presented by the APC in association with class I and class II MHC molecules. These immunogenic peptides interact with the corresponding T-cell receptors (TCR) and activate the multiple TAA-specific cytotoxic and helper T-cell clones that mediate a protective anti-tumour immune response.
