Abstract
CDK6 is an oncogenic kinase regulating the cell cycle. In this issue of Cancer Cell, Kollmann and colleagues demonstrate that CDK6 performs kinase-independent transcriptional function in regulating expression of VEGF-A and p16INK4a. These observations link the cell cycle machinery and angiogenesis, and reveal the presence of a fail-safe anti-proliferative mechanism.
Uncontrolled cell proliferation is a hallmark of cancer. Many cellular oncogenes have been shown to promote cell proliferation by affecting a variety of signaling pathways that impinge on the G1→S progression of the cell cycle. Similarly, components of the cell cycle machinery are subject to genomic alterations, point mutations, or epigenetic modifications in human cancers. Among those, genes encoding D-type cyclins (D1, D2, D3) and their kinase partners (CDK4 and CDK6) are frequently amplified and overexpressed in many cancer types. Moreover, the inhibitor of cyclin D-CDK4/6 kinases, p16INK4a (together with p14ARF) represents the most frequently deleted locus across all human cancer types (Beroukhim et al., 2010). Therefore, it is currently well accepted that deregulated cyclin D-CDK4/6 kinase activity represents a driving force in neoplasia. Inhibitors of CDK4 and CDK6, PD0332991, LEE011, and LY2835219 have entered the clinics and are being used in cancer trials. The initial results are very promising. Thus, it was recently reported that treatment with PD0332991 greatly extended progression-free survival in women with advanced estrogen receptor-positive breast cancer.
At the molecular level, cyclin D-CDK4/6 complexes drive cell proliferation by at least two distinct mechanisms. During cell cycle progression CDK4 and CDK6 kinases phosphorylate the retinoblastoma protein (pRB) and pRB-related p107 and p130 proteins. Phosphorylation of these proteins leads to liberation or de-repression of E2F transcription factors, which then induce genes necessary for S-phase entry and progression (Figure 1). Furthermore, cyclin D-CDK4/6 complexes phosphorylate transcription factors, such as SMAD2/3 and FOXM1. In addition to these kinase-dependent roles, cyclin D-CDK4/6 complexes also bind to the CDK inhibitors p21Cip1 and p27Kip1 and sequester them away from cyclin E-CDK2 kinase. This leads to activation of the cyclin E-CDK2 kinase activity, which further promotes cell cycle progression by phosphorylating a wide range of cellular proteins (Figure 1).
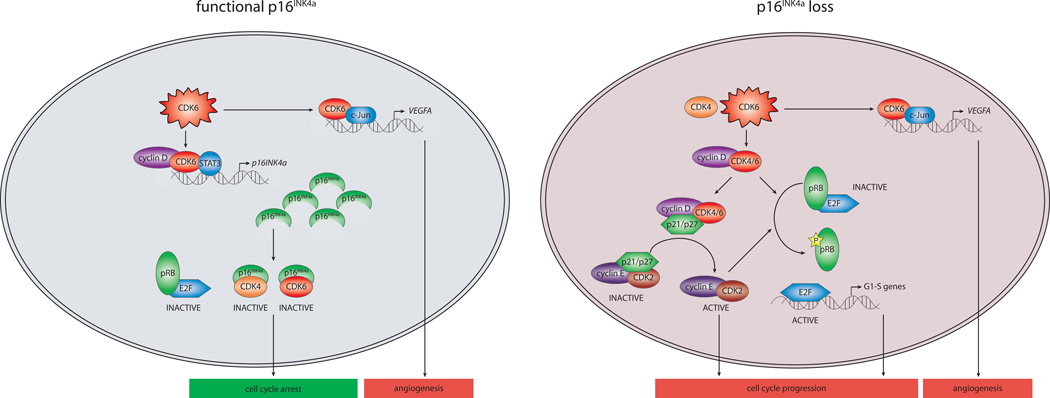
Figure 1. Model of the Function of CDK6 as Regulator of Cell Cycle and Angiogenesis.
Regulation of cell cycle and proliferation by CDK6 depends on the functionality of the p16INK4a protein. CDK6 overexpression can either cause cell cycle arrest in a cell with functional p16INK4a by activating p16INK4a transcription in a kinase-independent manner (left) or promote cell cycle progression if p16INK4a function is lost (right). In addition to regulating cell cycle, CDK6 also induces angiogenesis by activating transcription of VEGF-A, a known angiogenic factor, in a kinase-independent manner.
Genetic experiments revealed that knockout mice devoid of CDK4 or CDK6 are viable and develop relatively normally, indicating that these proteins are dispensable for normal proliferation of the majority of cell types (Malumbres et al., 2004). Some specific compartments, however, displayed proliferative deficits, consistent with a positive, growth-promoting function for CDK4 and CDK6. Importantly, CDK4- and CDK6-null mice were shown to be resistant to specific cancer types (Hu et al., 2009). Moreover, acute ablation of CDK4 or acute inhibition of CDK4/6 kinase activity in mouse cancer models blocked progression of cancers and leukemias by triggering cancer cell senescence or apoptosis (Puyol et al., 2010; Choi et al., 2012; Sawai et al., 2012). All these observations firmly established CDK4 and CDK6 as growth-promoting proteins.
The study of Kollmann et al. published in this issue of Cancer Cell reveals several novel and unexpected roles for CDK6 (Kollmann et al., 2013). The authors show that - contrary to what one might expect from a positive regulator of the cell cycle engine - overexpression of CDK6 inhibits cell cycle progression and proliferation of BCR-ABL transformed B-cell leukemia/lymphoma cells. Consistent with this observation, the authors demonstrate that CDK6 overexpression delays tumor formation upon injection of leukemic cells into recipient mice, indicating that CDK6 can act to constrain cell proliferation in vivo. The authors explain this antiproliferative function of CDK6 by demonstrating that CDK6 induces high levels of p16INK4a, an inhibitor of CDK4/6 kinase and an inducer of cell cycle arrest (Figure 1, left). In contrast, in a mouse model of T-cell lymphoma in which p16INK4a is inactivated by promoter methylation, CDK6 performs its known growth-promoting function. This implicates that in order to fulfill its oncogenic role, overexpressed CDK6 requires cancer cells to silence p16INK4a (Figure 1, right). Consistent with this prediction, Kollmann et al. observed an inverse correlation between CDK6 and p16INK4a levels in human B-cell and T-cell lymphomas. Thus, induction of p16INK4a by CDK6 serves as a fail-safe mechanism that restricts excessive activity of CDK6 by forming a negative feedback loop.
The second lesson from the study of Kollmann et al. is that CDK6, in addition to its cell cycle role, can also control formation of blood vessels in lymphoid tumors. The authors demonstrate that overexpressed CDK6 induces transcription of vascular endothelial growth factor A (VEGFA), a known angiogenic factor that stimulates the formation of new blood vessels by endothelial cells. This unexpected observation reveals that overexpressed CDK6 can both drive cell cycle progression (in the absence of p16INK4a) and induce angiogenesis, thereby linking two hallmark cancer features. The combined promotion of cellular proliferation and angiogenesis resembles the characteristics of some other known oncogenes, most notably c-Myc (Dews et al., 2006). It remains to be seen whether CDK6 also plays pro-angiogenic role in non-lymphoid tumors and whether other CDKs (possibly CDK4) might also perform such a function in other tumor types.
Intriguingly, the authors demonstrate that upregulation of p16INK4a and VEGF is caused by a direct, kinase-independent transcriptional mechanism played by CDK6. Specifically, CDK6 is shown to bind to p16INK4a and VEGFA promoters and to activate their expression through interaction with specific transcription factors (Figure 1). Earlier studies demonstrated that D-type cyclins play kinase-independent roles in transcription by acting at gene promoters. D-type cyclins were shown to interact with sequence specific transcription factors, such as STAT3 (Bienvenu et al., 2001), and to help to recruit chromatin-modifying enzymes, thereby influencing gene expression (Fu et al., 2004). Consistent with these findings, Kollmann et al. detected the presence of cyclin D2, together with CDK6, at p16INK4a and VEGFA promoters. Moreover, the transcriptional activation of p16INK4a by CDK6 requires the presence of D-type cyclins and depends on transcription factor STAT3. In contrast, induction of VEGF-A transcription does not require D-type cyclins and operates via AP-1 family transcription factor c-Jun. It remains to be seen whether another protein brings CDK6 to the transcriptional machinery in this setting or, alternatively, CDK6 interacts directly with c-Jun. The involvement of CDK6 in controlling transcription without D-type cyclins is unexpected, as previously transcriptional functions were ascribed to D-type cyclins, but not to their CDK partners. However, a report has proposed a transcriptional function for CDK6 in regulating androgen-dependent expression of prostate specific antigen (Lim et al., 2005).
Another unexpected outcome of the current study is that the observed roles for CDK6 are not shared by a closely related kinase, CDK4. CDK4 and CDK6 display a high degree of homology and are though to act in a largely redundant fashion. It remains to be seen whether CDK4 can also perform cyclin D-independent transcriptional functions, perhaps by acting at different sets of promoters. Global approaches, such as ChIP-Seq, will allow the comparison of the interaction of CDK4, CDK6, and D-type cyclins with the genome in an unbiased, genome-wide fashion.
Currently, the roles of CDK4 and CDK6 in human cancer are believed to be mainly kinase-dependent, a notion that led to development of specific CDK4/6 inhibitors which are now in clinical trials. These inhibitors are expected to inhibit the proliferative function of CDK4/6 complexes, leading to cell cycle arrest and potentially triggering senescence or apoptosis. However, the novel kinase-independent role of CDK6 in promoting angiogenesis suggests that a new class of CDK4/6 inhibitors capable of interrupting the angiogenic function, in addition to blocking the proliferative role of CDK6, might be clinically more successful. These inhibitors might also be useful in pRB-deficient tumors, in which CDK6 inhibition is not expected to affect cell proliferation.
ACKNOWLEDGEMENTS
We apologize to colleagues whose work we were unable to cite due to space limitation. Our work is supported by R01 CA108420 from NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, et al. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienvenu F, Gascan H, Coqueret O. J. Biol. Chem. 2001;276:16840–16847. doi: 10.1074/jbc.M100795200. [DOI] [PubMed] [Google Scholar]
- Choi YJ, Li X, Hydbring P, Sanda T, Stefano J, Christie AL, Signoretti S, Look AT, Kung AL, von BH, Sicinski P. Cancer Cell. 2012;22:438–451. doi: 10.1016/j.ccr.2012.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dews M, Homayouni A, Yu D, Murphy D, Sevignani C, Wentzel E, Furth EE, Lee WM, Enders GH, Mendell JT, Thomas-Tikhonenko A. Nat. Genet. 2006;38:1060–1065. doi: 10.1038/ng1855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu M, Wang C, Li Z, Sakamaki T, Pestell RG. Endocrinology. 2004;145:5439–5447. doi: 10.1210/en.2004-0959. [DOI] [PubMed] [Google Scholar]
- Hu MG, Deshpande A, Enos M, Mao D, Hinds EA, Hu GF, Chang R, Guo Z, Dose M, Mao C, et al. Cancer Res. 2009;69:810–818. doi: 10.1158/0008-5472.CAN-08-2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kollmann K, Heller G, Schneckenleithner C, Warsch W, Scheicher R, Ott RG, Schäfer M, Fajmann S, Schlederer M, Schiefer AI, et al. Cancer Cell. 2013;24 doi: 10.1016/j.ccr.2013.07.012. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim JT, Mansukhani M, Weinstein IB. Proc. Natl. Acad. Sci. U. S. A. 2005;102:5156–5161. doi: 10.1073/pnas.0501203102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malumbres M, Sotillo R, Santamaria D, Galan J, Cerezo A, Ortega S, Dubus P, Barbacid M. Cell. 2004;118:493–504. doi: 10.1016/j.cell.2004.08.002. [DOI] [PubMed] [Google Scholar]
- Puyol M, Martin A, Dubus P, Mulero F, Pizcueta P, Khan G, Guerra C, Santamaria D, Barbacid M. Cancer Cell. 2010;18:63–73. doi: 10.1016/j.ccr.2010.05.025. [DOI] [PubMed] [Google Scholar]
- Sawai CM, Freund J, Oh P, Ndiaye-Lobry D, Bretz JC, Strikoudis A, Genesca L, Trimarchi T, Kelliher MA, Clark M, Soulier J, Chen-Kiang S, Aifantis I. Cancer Cell. 2012;22:452–465. doi: 10.1016/j.ccr.2012.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]