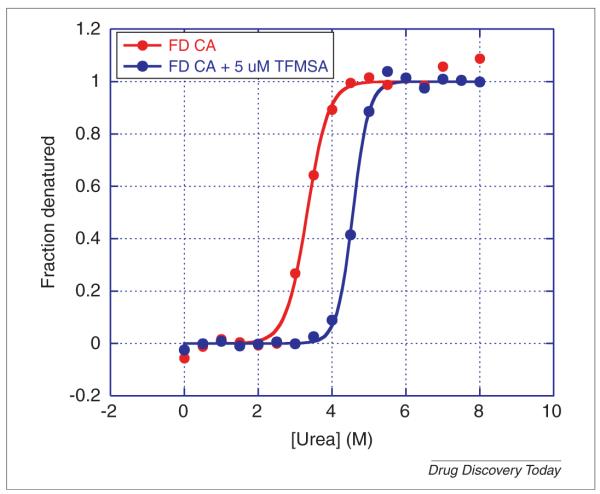
FIGURE 7.
Urea denaturation of carbonic anhydrase in the absence and presence of 5 μm of the inhibitor trifluoromethanesulfonamide (TFMSA). Carbonic anhydrase, isoform I, from human erythrocytes was dissolved in 25 mm MES, pH 6.0 at a concentration of 10 μm (0.3 mg/mL). Two sets of 17 test tubes were prepared containing 180 μL of MES with a gradient of 0.0–8.0 m urea (final concentration). DMSO (2 μL) was added to one of the series of test tubes and 2 μL of 500 μm TFMSA dissolved in DMSO was added to the other series for a final concentration of 5 μm TFMSA. The protein was dispensed in aliquots of 20 μL into the test tubes and incubated for four hours at 25°C. The final concentration of protein was 1 μm (0.03 mg/mL). The excitation wavelength was 280 nm and the emission wavelength 355 nm. Fluorescence measurements were performed manually in a Cary Eclipse spectrofluorometer (Varian, Agilent).