Figure 3.
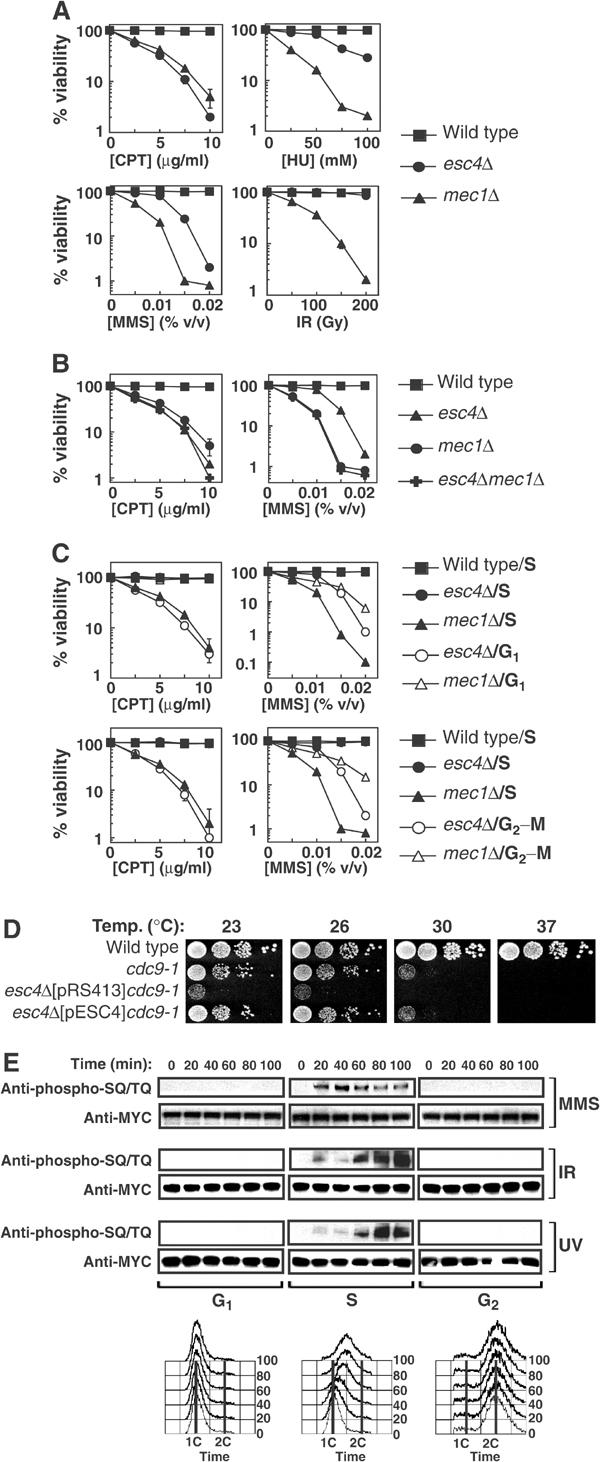
Esc4p responds to DNA damage specifically during DNA replication. (A, B) Strains W303-1A (wild-type), JRY99 (esc4Δ), U960-5C (mec1Δ sml1-1) (A, B) and JRY103 (esc4Δmec1Δ sml1-1) (B) were grown to early log phase in liquid culture. The relevant drug was added at the indicated concentrations in the case of CPT, MMS and HU, and the cells were grown at 30°C for a further 3.5 h. Cell suspensions were diluted 100-fold, plated on YPD-agar and incubated for 3 days at 30°C before counting viable colonies. In the case of IR, diluted cells were irradiated with a Cs137 source at a delivery rate of 2.8 Gy/min, before spreading on YPD-agar. The average of five independent experiments is shown. (C) Strains W303-1A (wild-type), JRY99 (esc4Δ) and U960-5C (mec1Δsml1Δ) were grown to early log phase and arrested in G1 by addition of alpha-factor (5 μg/ml). Cells were then released from arrest into fresh medium (‘S') and incubated for 10 min at 30°C before addition of CPT or MMS at the indicated concentrations for 120 min. Alternatively, cells were incubated in fresh medium containing alpha-factor to hold cells in G1 during treatment with CPT and MMS (‘G1'). In other experiments, cells were held at the G2–M boundary by incubation with nocodazole (15 μg/ml) for 1.5 h before addition of CPT or MMS in the continued presence of nocodazole (‘G2–M'). After exposure to genotoxins, cells were washed and spread on YPD-agar and colonies were counted 3 days later. (D) Strains A364a (wild-type), DLY1080 (cdc9-1) and JRY102 (esc4Δcdc9-1) transformed with pRS413 (empty vector) or with pESC4 were grown to mid-log phase and 10-fold serial dilutions were spotted on YPD-agar and incubated at the indicated temperature for 2–4 days before the number of viable colonies was determined. (E) Cells expressing MYC(13)-Esc4p (strain JRY100) were grown to mid-log phase, arrested in G1 and released into S phase in the presence of MMS (0.02% v/v) for the times indicated or maintained in alpha-factor to hold cells in G1 during MMS treatment. Alternatively, cells were arrested at the G2–M boundary by addition of nocodazole and held there during treatment with MMS. Cells were also exposed to UV light (50 J/m2) or IR (150 Gy) and incubated at 30°C for the times indicated while held in G1 or at G2–M or released into S phase. Native extracts were prepared and anti-MYC immunoprecipitates were subjected to Western blot analysis. Samples of cells were also fixed in 70% ethanol and subjected to FACS analysis.
