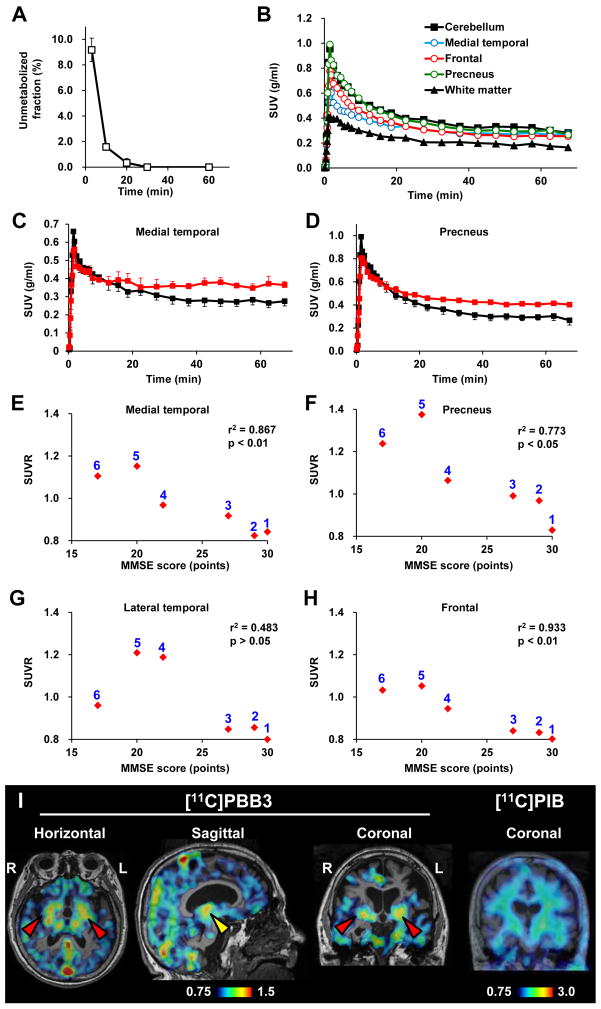
Figure 9. Pharmacokinetic profiles of [11C]PBB3 administered to humans and PET images of a patient clinically diagnosed as having corticobasal syndrome.
(A) Time course of unmetabolized [11C]PBB3 fraction in plasma following intravenous radiotracer injection. The plot was generated by averaging data from 6 individuals. (B) Time-radioactivity curves in different brain regions of cognitively normal control subjects over 70 min after intravenous injection of [11C]PBB3. Data were generated by averaging values in two individuals, and are presented as standard uptake values (SUVs). (C, D) Comparisons of time-radioactivity curves in the medial temporal region (C) and precuneus (D) of normal controls (black symbols and lines; n = 3) and AD patients (red symbols and lines; n = 3). (E–H) Scatterplots illustrating correlation of SUVRs with MMSE scores in the medial temporal region (E), precuneus (F) and lateral temporal (G) and frontal (H) cortices. Numbers beside symbols denote subject ID as indicated in Fig. 8. Coefficients of determination (r2) and p values by t-test are displayed in graphs. (I) [11C]PBB3- and [11C]PIB-PET images in a subject with clinical diagnosis of corticobasal syndrome. Images were generated as in Figs. 7 and 8. Accumulation of [11C]PBB3 was noticeable in the basal ganglia (red arrowheads) with right-side dominance and an area containing the thalamus and midbrain (yellow arrowhead).