Figure 7.
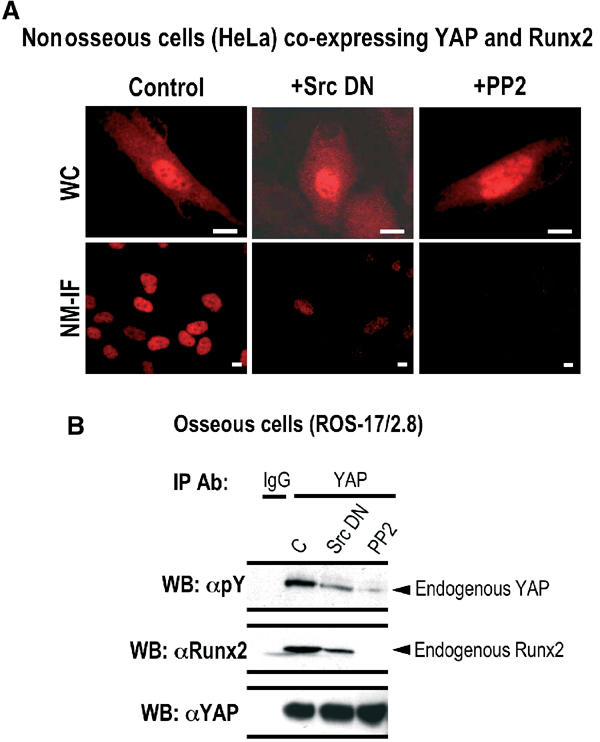
Tyrosine phosphorylation of YAP regulates its interaction with Runx2 and subsequent subnuclear trafficking. (A) HeLa cells, co-expressing YAP and Runx2, were transfected with Src DN or treated with PP2 (5 μM) for 1 h and WC or NM-IF preparations and in situ immunofluorescence microscopy. Inhibition of either Src tyrosine kinase (+Src DN) or its family members (+PP2) does not alter subcellular localization of YAP. Subnuclear trafficking of YAP is severely compromised when the kinase activity of Src alone or its family members is inhibited. (B) To directly assess the effects of Src DN or PP2 on tyrosine phosphorylation of endogenous YAP and its interaction with native Runx2, endogenous YAP was immunoprecipitated from ROS 17/2.8 cells. The immunoprecipitates were resolved by SDS–PAGE and subjected to western blot analysis. The tyrosine phosphorylation of YAP was determined by a mouse monoclonal phospho-specific antibody (Py; 1:2000) raised against phospho-tyrosine (top panel). The blot was stripped and re-probed with a mouse monoclonal antibody against Runx2 to assess the presence of endogenous Runx2 in the immunoprecipitates (middle panel). The efficiency of YAP immunoprecipitation was assessed by a YAP rabbit polyclonal antibody (bottom panel).
