Abstract
The results of two independent, randomized, two-period crossover, single-center studies, conducted to assess the pharmacokinetics of ticagrelor ± aspirin, inhibition of platelet aggregation (IPA) with ticagrelor/aspirin vs. clopidogrel/aspirin, and safety, tolerability, and bleeding times are reported here. In Study A (open-label), 16 volunteers received ticagrelor (50 mg bid Days 1–5; 200 mg bid Days 6–9; one 200 mg dose on Day 10) ± 300 mg qd aspirin (Days 1–10). In Study B (double-blind, double-dummy), 16 volunteers received aspirin (300 mg loading dose/75 mg qd Days 2–9) with either ticagrelor (200 mg bid Days 4–8, one 200 mg dose on Day 9) or clopidogrel (300 mg loading dose Day 4, 75 mg qd Days 5–9). At steady-state ticagrelor (50 mg bid, or 200 mg bid), concomitant aspirin (300 mg qd) had no effect on mean maximum plasma concentration (C max), median time to C max (t max), or mean area under the plasma concentration-time curve for the dosing interval (AUC0– τ) for ticagrelor and its primary metabolite, AR-C124910XX. Following 200 mg bid ticagrelor, mean C max and AUC0– τ for both parent and metabolite were comparable with co-administration of aspirin at 75 mg and 300 mg qd. Aspirin (300 mg qd) had no effect on IPA (ADP-induced) by ticagrelor. However, aspirin and ticagrelor had an additive effect on IPA (collagen-induced). Ticagrelor/aspirin increased bleeding times vs. baseline. Ticagrelor/aspirin co-administration was well tolerated at all dose combinations evaluated. In summary, the findings of this study demonstrate that co-administration of aspirin (300 mg qd) with ticagrelor (50 mg bid, or 200 mg bid) had no effect on ticagrelor pharmacokinetics or IPA (ADP-induced) by ticagrelor.
Keywords: Ticagrelor, P2Y12 receptor antagonist, antiplatelet therapy, aspirin, clopidogrel, pharmacokinetics
Introduction
Ticagrelor is a direct-acting, reversibly binding, oral P2Y12 receptor antagonist [1]. In patients with acute coronary syndromes (ACS), the Phase III PLATelet inhibition and patient Outcomes (PLATO) trial demonstrated that ticagrelor plus aspirin significantly reduced the primary composite endpoint of myocardial infarction (MI)/stroke/death from vascular causes vs. clopidogrel plus aspirin, without increasing PLATO-defined major bleeding [2]. Ticagrelor is approved in more than 70 countries for the prevention of atherothrombotic events in adult ACS patients [3], [4]. Updated guidelines recommend ticagrelor co-administered with low-dose aspirin as a dual antiplatelet therapy for the management of any ACS patient [5] and those with non-ST-segment elevation [6]. Ticagrelor is also recommended as an oral antiplatelet therapy in percutaneous coronary intervention [7].
Ticagrelor underwent an extensive development program which included early pharmacokinetic and pharmacodynamic studies. Ticagrelor has a major metabolite, AR-C124910XX [8], which is approximately equipotent to ticagrelor in blocking ADP-induced platelet aggregation (AstraZeneca, data on file). Rapid onset of inhibition of platelet aggregation (IPA) is achieved with ticagrelor, and IPA then declines from 12 hours post-dosing [9]. Ticagrelor plus aspirin showed more rapid, greater and more consistent IPA vs. clopidogrel plus aspirin in patients with ACS [10] or atherosclerosis [11].
Dual antiplatelet therapy involving aspirin is a key strategy for managing ACS [6]. By irreversibly inhibiting cyclooxygenase, aspirin blocks arachidonic-acid induced platelet aggregation [12]. A single, oral 100 mg aspirin dose is sufficient to completely block the cyclooxygenase activity [13]. However, a wide range (50–1500 mg once daily [qd]) of aspirin doses has been used in clinical trials [14–16]. Also, several analyses have reported that aspirin efficacy (i.e. prevention of serious vascular events) is not dose-related, and the highest efficacy is seen with 75–150 mg qd [14], [17]. In contrast, bleeding events increase with increasing aspirin dose [18].
Aspirin dose has also been shown to influence the efficacy of ticagrelor. A post-hoc subgroup analysis of the PLATO trial showed a significant interaction (p = 0.00006) between aspirin dose category and treatment. In patients taking ≤100 mg maintenance aspirin, ticagrelor was associated with a reduction in the rate of MI, stroke and death from vascular causes, compared with clopidogrel (adjusted hazard ratio [HR] 0.77 [95% confidence interval (CI), 0.69–0.86]). However, in patients taking a maintenance aspirin dose ≥300 mg, the HR of 1.45 (95% CI, 1.01–2.09) favored clopidogrel [19].
Given the key role of aspirin in ACS therapies, two healthy volunteer studies were conducted early in the ticagrelor development program to explore the potential effect of aspirin on the pharmacokinetics and pharmacodynamics of ticagrelor. Two aspirin doses were assessed in these previously unpublished trials: 75 mg qd (in the effective dose range for thromboembolic event reduction [14]) and 300 mg qd (close to 325 mg qd commonly used in the USA [17]). The primary objectives of these studies were to evaluate ticagrelor pharmacokinetics with and without aspirin (D5130C0005; Study A), and to compare IPA with ticagrelor plus aspirin vs. clopidogrel plus aspirin (D5130C05261; Study B). Key secondary objectives included the assessment of the safety and tolerability of ticagrelor with or without aspirin, and bleeding times.
The data presented in these studies provide information important to the understanding of pharmacokinetic and pharmacodynamic aspects of any potential interaction between ticagrelor and aspirin maintenance dose.
Methods
Study populations
Sample size
Sixteen randomized, healthy volunteers were planned for Study A to achieve at least 12 evaluable subjects, which would give at least 80% power (5% significance level) to show that the 90% CIs for differences between ticagrelor/aspirin and ticagrelor were within 70–143%, if the hypothesis of equality was true.
For study B, a non-parametric calculation (at least 90% power, 5% significance level) showed that at least 12 healthy volunteers were required if the probability of a higher IPA in the ticagrelor/aspirin group vs. the clopidogrel/aspirin group was to be 90%.
Inclusion/exclusion criteria
For both studies, key inclusion criteria were: males or females (post-menopausal or surgically sterile); 18–65 years; body mass index (BMI) 18–30 kg/m2. Key exclusion criteria were: presence or history of conditions affecting drug disposition; any clinically significant electrocardiogram (ECG) findings, laboratory or coagulation abnormalities; a history of alcohol or substance abuse.
Ethics
Written, informed consent was provided by all volunteers in both studies. An Independent Ethics Committee for each study approved the final protocol and amendments. The studies were performed in accordance with Declaration of Helsinki principles, and were consistent with ICH/Good Clinical Practice, applicable regulatory guidelines and AstraZeneca bioethics policy.
Study designs and treatment
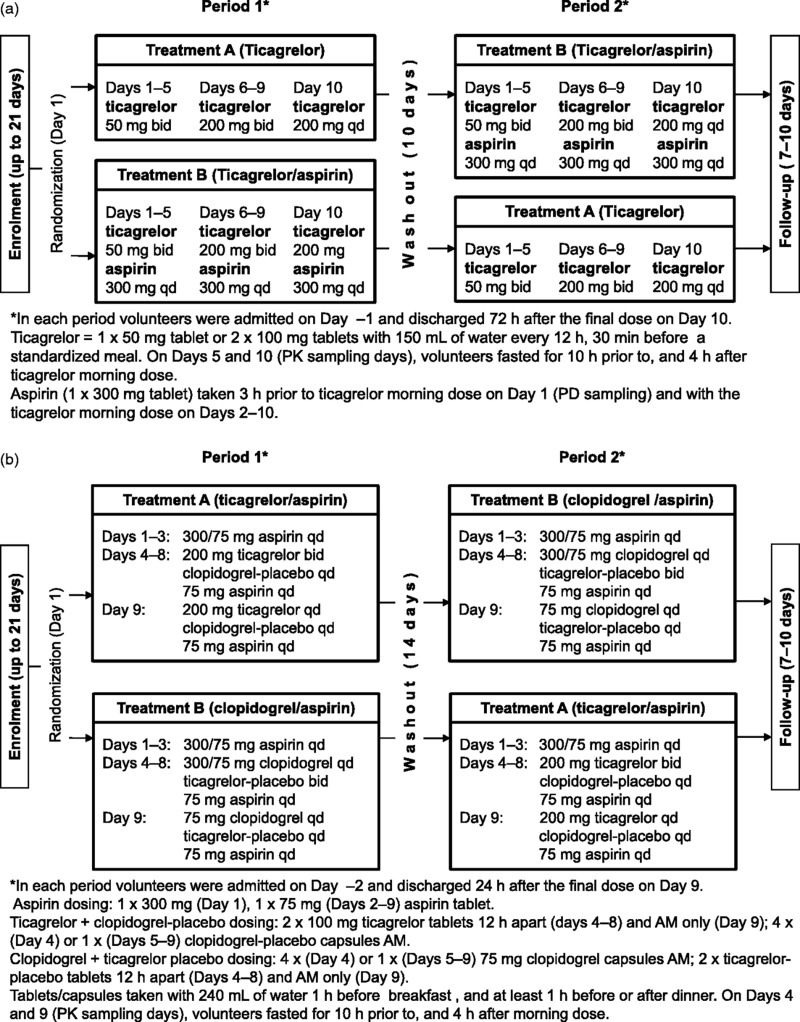
Study A was a randomized, open-label, two-period crossover, single center study (Figure 1a). Enrolment and screening took place up to 21 days prior to admission. Eligibility was confirmed on admission (Day –1) and on study Day 1 volunteers were randomized to one of the two treatment sequences (AB or BA). Volunteers (N = 16) received ticagrelor 50 mg (twice daily) bid Days 1–5, 200 mg bid Days 6–9, and a single 200 mg dose on Day 10 without aspirin (Treatment A in Study A) and with aspirin 300 mg qd Days 1–10 (Treatment B in Study A). The dosing schedule and details are shown in Figure 1(a). Caffeine-containing products, alcohol, over-the-counter preparations, and non-steroidal anti-inflammatory drugs were not permitted from prior to admission until discharge after each period.
Figure 1.
Designs for Study A and Study B.
Study B was a randomized, double-blind, double-dummy, two-treatment, two-period crossover, single center study (Figure 1b). Enrolment and screening took place up to 21 days prior to admission. Eligibility was confirmed on admission (Day –2), and on study Day 1 volunteers were randomized to one of two treatment sequences (AB or BA). Volunteers (N = 16) received ticagrelor at 200 mg bid on Days 4–8 and in the morning only on Day 9 (Treatment A in Study B), and clopidogrel at a 300 mg loading dose on Day 4, then 75 mg qd Days 5–9 (Treatment B in Study B). For both treatments A and B, aspirin was administered on Day 1 (300 mg), and on Days 2–9 75 mg qd. The dosing schedule and details are shown in Figure 1(b). This study used the early tablet formulation of ticagrelor; 100 mg bid was demonstrated to provide comparable exposure to that of 90 mg bid of the commercially-available tablet formulation. Restrictions during the study were as for Study A.
Ticagrelor pharmacokinetic sample collection
Venous blood samples (2 ml) were collected into lithium-heparin tubes. Plasma samples were prepared by centrifugation (1500 g, 10 minutes, 4°C) within 30 minutes, and stored frozen (−20°C) until analyzed.
For Study A, sampling times were: pre-dose on Days 1, 4, and 9; 0 (pre-dose), 0.5, 1, 2, 3, 4, 6, 8, 10, and 12 hours post-morning dose on Days 5 (ticagrelor 50 mg bid) and 10 (ticagrelor 200 mg bid), and 18, 24, 36, 48, and 72 hours post-morning dose on Day 10. Sampling times for Study B following multiple doses of ticagrelor/aspirin and clopidogrel/aspirin were 0 (pre-dose), 0.5, 1, 2, 3, 4, 6, 8, 10, 12, 18, and 24 hours post-morning dose on the final day of dosing (Day 9).
Analysis of ticagrelor and AR-C124910XX
Ticagrelor and AR-C124910XX plasma concentrations were analyzed, after protein precipitation, using a fully validated liquid chromatography with the tandem mass spectrometry method [20]. Lower limits of quantification were 5 ng/ml (ticagrelor) and 2.5 ng/ml (AR-C124910XX).
Assessment of platelet aggregation inhibition
Blood sampling times in Study A for both study periods were: Day 1 pre-dose (baseline), and 2 hours post-aspirin dose on Day 1 (Treatment B only); 0 (pre-dose), 4, 8, 12 hours post-morning dose on Day 5; 0 (pre-dose), 2, 4, 8, 12, 24, and 36 hours post-morning dose on Day 10. For Study B, sampling times were: pre-dose on Day 1 (baseline); 2 hours post-aspirin dose on Day 3; 0 (pre-dose) on Day 4; 0 (pre-dose), 2, 4, 12, and 24 hours post-morning dose on Day 9.
Venous blood samples (4.5 ml) were collected into tubes containing 0.5 ml of 3.8% sodium citrate. Samples were centrifuged (180–200 g, 10 minutes, room temperature). The platelet count of the resulting platelet-rich plasma was adjusted to 300 × 109/l using autologous platelet-poor plasma. Agonists for platelet aggregation were 20 µM ADP, or 4 µg/ml collagen. Final-extent IPA was assessed by optical aggregometry as described previously [21].
Evaluation of bleeding times
Simplate method
Bleeding times in Study A for both study periods were: Day 1 pre-dose; 4 and 24 hours post-morning dose on Days 4 and 9. A distending venous pressure (40 mmHg) was applied with a standard sphygmomanometer cuff for 30–60 seconds before and throughout the procedure. A Simplate® device was used to make one incision (1 mm depth by 5 mm long) on the forearm. The incision was blotted every 30 seconds until bleeding ceased, and the time recorded to the nearest 15 seconds.
Lancet method
In Study A, lancet bleeding times were measured at enrolment, and at 4 hours post-dosing on Day 9 of both periods. For Study B, bleeding times were assessed at screening (baseline): 4 hours post-aspirin dose on Day 3, pre-dose on Day 4, pre-dose and 4 hours post-dose on Day 9 of both study periods.
A distending venous pressure (40 mmHg) was applied with a standard sphygmomanometer cuff for 30 seconds before and throughout the procedure. Using a standard lancet (Styrex 3 mm depth by 1 mm wide [Study A]; BD Genie Safety Lancet, 2 mm depth by 1.5 mm width [Study B]), two evenly spaced punctures on the circumference of a 20 mm diameter circle were made rapidly into the volar surface of the forearm away from superficial veins. Blood was blotted from the punctures every 15 seconds (Study B) or 30 seconds (Study A) until bleeding ceased, and the time to the nearest 15 seconds was recorded [22].
Safety and tolerability
Safety was evaluated by monitoring adverse events (AEs), laboratory parameters, vital signs, ECGs, physical examination at various times throughout the treatment periods and at the follow-up visit.
Data analyses
Pharmacokinetic analyses
Pharmacokinetic parameters were estimated by standard non-compartmental methods using WinNonlin Professional (Pharsight Corporation, Mountain View, California). Maximum concentration (C max), time to C max (t max), and area under the plasma concentration-time curve for the dosing interval (AUC0– τ) were calculated for ticagrelor and AR-C124910XX.
Pharmacodynamic analyses
IPA (%) was calculated by 100 × (PABL–PAT)/PABL; PABL = mean pre-dose baseline response, PAT = mean platelet aggregation response at time T. For Study B, individual peak IPA (IPAmax) was the highest observed IPA following ticagrelor or clopidogrel administration, and time to IPAmax (TIPAmax) was evaluated. Values for area under the effect curve (AUEC) of IPA from 0 to 12 hours (AUEC0–12) and from 0 to 24 hours (AUEC0–24) were calculated from IPA-time curves, using the linear trapezoidal rule.
Statistical analyses
Statistical analyses were performed using SAS Version 8.12 (SAS Institute, Cary, North Carolina, USA). Pharmacokinetic data were descriptively summarized. Plasma concentration-time curves were plotted. In Study A, for the pharmacokinetic parameters, C max and AUC0– τ, 90% CIs were calculated for the differences between ticagrelor/aspirin and ticagrelor alone. After log-transformation, these data were analyzed by the analysis of variance (ANOVA) fitting a fixed-effect term for volunteers. Least square (LS) mean ratio point estimates and two-sided 90% CIs for ticagrelor/aspirin:ticagrelor ratio were exponentially back-transformed to present the point estimates and CIs in the original linear scale.
In Study B, mean IPA-time curves were plotted. AUEC0–12, AUEC0–24, and IPAmax data were analyzed by ANOVA, fitting fixed-effect terms for sequence, volunteer within sequence, period, and treatment. Point estimates and corresponding 95% CIs were estimated for the difference between ticagrelor/aspirin and clopidogrel/aspirin groups.
Results
Demographic and baseline characteristics
In Study A, of the 16 randomized volunteers, 14 (88%) were male, 15 were Caucasian, and one was Black. The mean (range) age and body weight were 37 (21–54) years and 73 (53–108) kg, respectively. Three volunteers discontinued due to AEs.
In Study B, 15/16 (94%) volunteers were male, 15 were Caucasian, and one was Oriental. The mean (range) age and body weight were 35 (18–53) years and 72 (55–97) kg, respectively. All volunteers completed this study.
Effect of aspirin on ticagrelor pharmacokinetic parameters
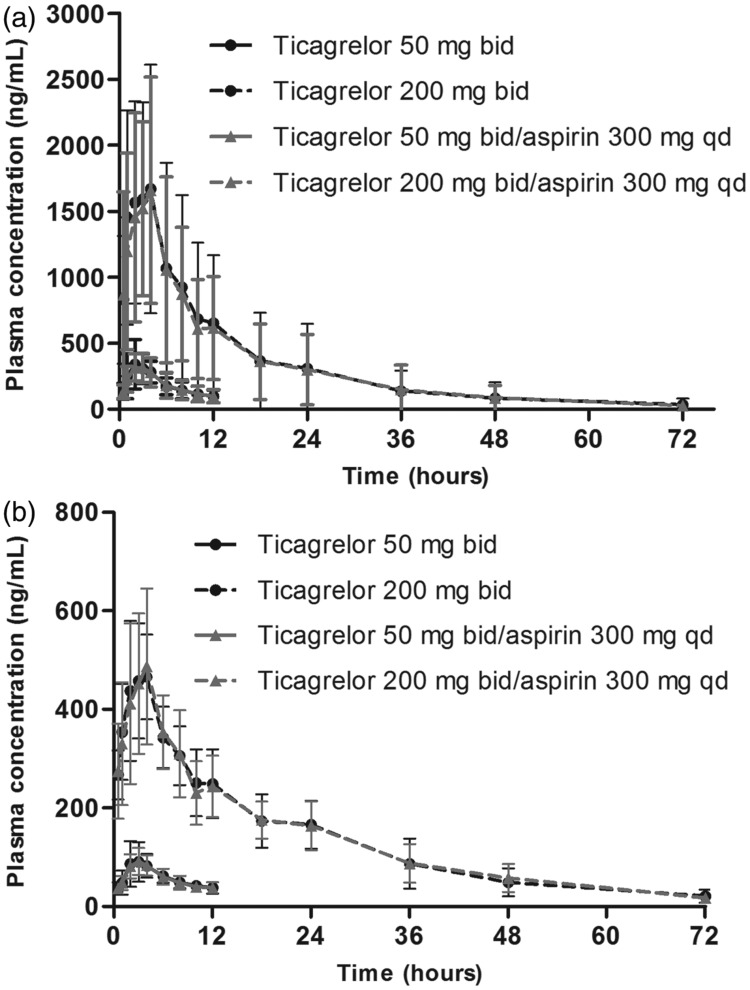
In Study A, following the administration of ticagrelor alone, ticagrelor was rapidly absorbed (Figure 2a) with a median t max of 3 hours, and AR-C124910XX was rapidly formed (Figure 2b) with a similar median t max (3 hours; Table I). Mean C max and AUC0– τ for AR-C124910XX were approximately one-third of ticagrelor values at both ticagrelor doses (Table I).
Figure 2.
Plasma concentration profiles of (a) ticagrelor and (b) AR-C124910XX following administration of ticagrelor (50 mg bid for 5 days, or 200 mg bid for 4 days then qd for 1 day) ± once-daily aspirin (300 mg) (Study A). Values are mean ± SD, n = 13 at each time point.
Table I. .
Steady state pharmacokinetic parameters of ticagrelor and AR-C124910XX following the administration of ticagrelor ± once-daily aspirin.
| Study A |
Study B | ||||||
|---|---|---|---|---|---|---|---|
| Ticagrelor 50 mg bida
|
Ticagrelor 200 mg bidb
|
Ticagrelor 200 mg bidc | |||||
| Parameter | Ticagrelor alone (n = 13) | Ticagrelor + aspirin 300 mg qd (n = 13) | LS means ratio % (90% CI) | Ticagrelor alone (n = 13) | Ticagrelor + aspirin 300 mg qd (n = 13) | LS means ratio % (90% CI) | Ticagrelor + aspirin 75 mg qd (n = 16) |
| Ticagrelor | |||||||
| C max (ng/ml) | 353 (41) | 334 (32) | 96 (80–115) | 1777 (48) | 1697 (47) | 96 (80–116) | 1478 (37) |
| AUC0– τ (ng·h/ml) | 2218 (36) | 2193 (38) | 99 (86–114) | 12 026 (60) | 11 576 (54) | 97 (84–111) | 10 391 (33) |
| t max (h) | 3.0 (2.0–4.0) | 3.0 (1.0–6.0) | NA | 3.0 (1.0–4.0) | 3.0 (2.0–6.0) | NA | 3.1 (1.1–4.2) |
| AR-C124910XX | |||||||
| C max (ng/ml) | 92 (40) | 97 (25) | 106 (92–122) | 490 (20) | 495 (29) | 102 (88–117) | 501 (27) |
| AUC0– τ (ng·h/ml) | 682 (29) | 678 (23) | 100 (90–111) | 4038 (20) | 3951 (26) | 98 (89–109) | 4134 (23) |
| t max (h) | 3.0 (2.0–6.0) | 3.0 (2.0–6.0) | NA | 3.0 (2.0–4.0) | 4.0 (2.0–6.0) | NA | 3.1 (2.0–4.2) |
Data are geometric mean (coefficient of variation) for C max, and AUC0– τ, and median (range) for t max. AUC0– τ = area under the plasma concentration-time curve for the dosing interval; bid = twice daily; C max = maximum plasma concentration; LS mean ratio = least squares mean for ticagrelor as a percentage of the least squares mean for ticagrelor/aspirin; NA = not applicable; qd = once daily; t max = time to reach peak or maximum concentration.
50 mg bid for 5 days.
200 mg bid for 4 days, then a single 200 mg dose for one day.
200 mg bid for 5 days, then a single 200 mg dose for one day.
The plasma concentration-time profiles of ticagrelor (Figure 2a) and AR-C124910XX (Figure 2b), and key pharmacokinetic parameters (Table I) were comparable following the administration of ticagrelor with or without 300 mg aspirin (Study A). The LS mean ratios were close to 100% for C max and AUC0– τ for both ticagrelor and AR-C124910XX (Table I).
Mean C max, mean AUC0– τ, and median t max for ticagrelor and AR-C124910XX following the co-administration of ticagrelor (200 mg bid) with aspirin (75 mg qd) (Study B) were comparable with data following ticagrelor (200 mg bid) administration with 300 mg qd aspirin (Study A; Table I).
Adenosine diphosphate (ADP)-(20 µM)-induced platelet aggregation
Study A: Effect of aspirin on ticagrelor-induced IPA
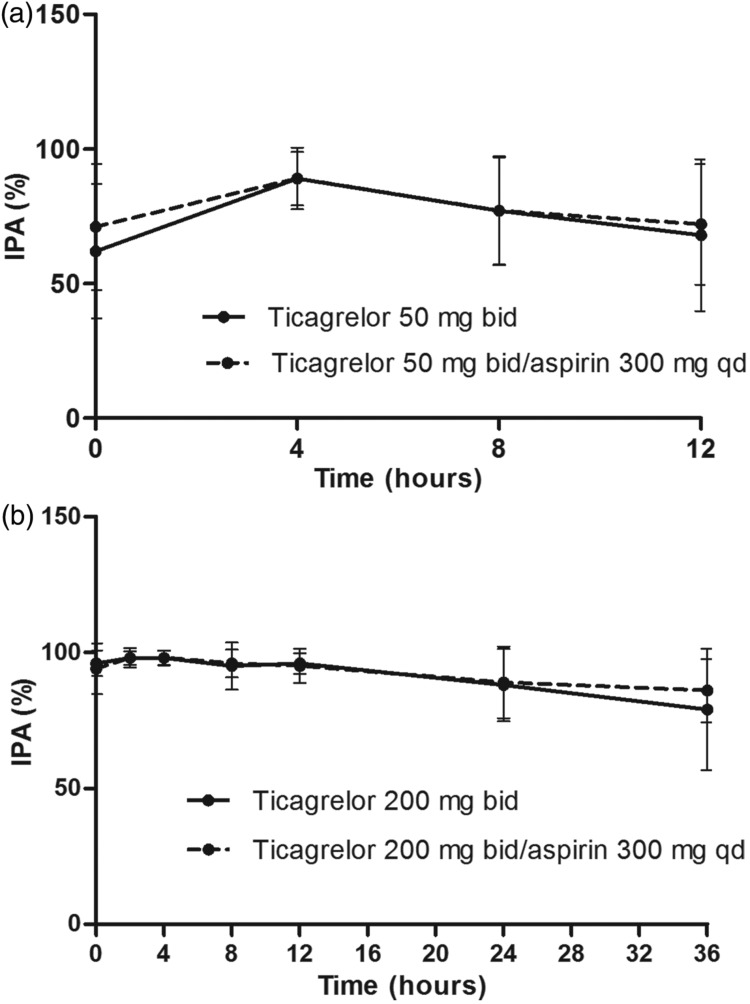
Mean final-extent IPA ranged from 62% to 89%, 0–12 hours after the last dose of 50 mg bid ticagrelor alone (Figure 3a). Almost complete inhibition of ADP-induced platelet aggregation was achieved with 200 mg bid ticagrelor (Figure 3b); mean IPA ranged from 95% to 98%, 0–12 hours post-dosing. For 24 and 36 hours post-dosing, the mean IPA declined to 88% and 79%, respectively.
Figure 3.
Inhibition of platelet aggregation (final-extent, 20 µM ADP-induced) following administration of ticagrelor (a) 50 mg bid for 5 days, and (b) 200 mg bid for 4 days then qd for 1 day, ± once-daily aspirin (300 mg) (Study A). Values are mean ± SD, n = 11 or 12 at each time point.
Co-administration of once-daily aspirin (300 mg) had no effect on final-extent IPA with either 50 mg bid (Figure 3a) or 200 mg bid (Figure 3b) ticagrelor. Mean IPA ranged from 71% to 89% and 94% to 98%, respectively, at 0–12 hours post-dosing.
Study B: Effect of aspirin on ticagrelor-induced IPA
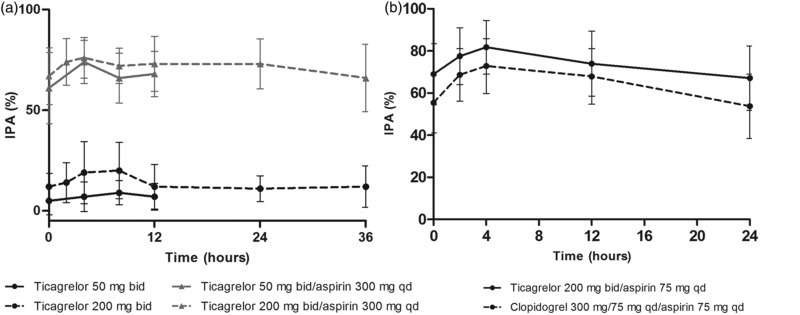
For ticagrelor (200 mg bid) co-administered with 75 mg aspirin daily, the mean final-extent IPA (ADP-induced) ranged from 91% to 99% at 0–12 hours post-dosing (Figure 4).
Figure 4.
Inhibition of platelet aggregation (final-extent, 20 µM ADP-induced) following administration of ticagrelor (200 mg bid for 5 days then a single 200 mg dose for 1 day) + aspirin (75 mg qd) and clopidogrel (loading dose 300 mg on Day 1 then 75 mg qd for 5 days) + aspirin (75 mg qd) (Study B). Values are mean ± SD, n = 16 at each time point.
Study B: Ticagrelor/aspirin vs. clopidogrel/aspirin
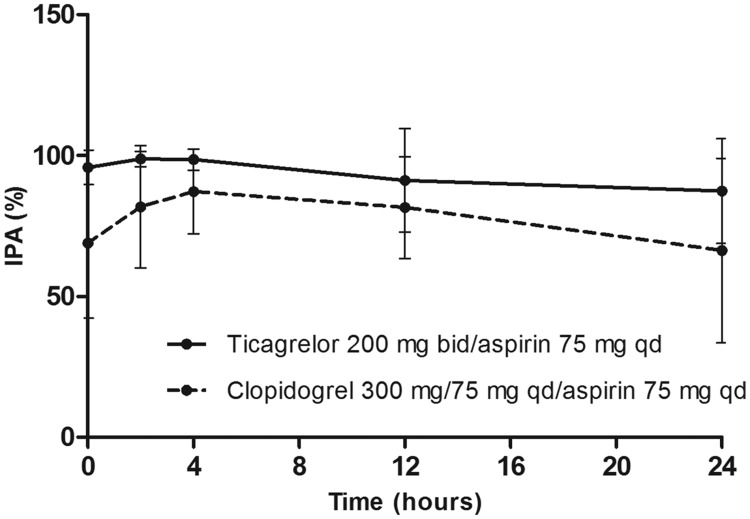
Ticagrelor/aspirin significantly increased mean final-extent IPA (ADP-induced; 95.8% [coefficient of variation (CV) 6.4%]) compared with aspirin alone (9.3% [CV, 112%]; difference, 86.5% [95% CI, <0.0001–90.3%]; p <0.0001).
At steady-state, mean final-extent IPA was higher with ticagrelor/aspirin compared with clopidogrel/aspirin at all time points evaluated (Figure 4). IPA response over the dosing interval remained high with ticagrelor/aspirin as pre-dose final IPA values were >80% in all ticagrelor-treated volunteers and in only 50% of clopidogrel-treated volunteers. Inter-individual variability of IPA parameters was lower with ticagrelor/aspirin vs. clopidogrel/aspirin (Figure 4, Table II).
Table II. .
IPA parameters at final-extent 20 µM ADP-induced and collagen-induced aggregation (Study B).
| Parameter | Ticagrelor 200 mg bida/aspirin 75 mg qd (n = 16) | Clopidogrel 300 mg load, 75 mg qdb/aspirin 75 mg qd (n = 16) | Difference (ticagrelor – clopidogrel) (95% CI) | p-value |
|---|---|---|---|---|
| ADP-induced | ||||
| IPAmax (%) | 99.5 (2) | 88.3 (18) | 11.1 (3.4–18.8) | 0.0077 |
| TIPAmax (h) | 0.0 (0.0–4.0) | 4.0 (0.0–12.0) | NA | NA |
| AUEC0–12 (%·h) | 1163 (10) | 1002 (25) | 161 (40–283) | 0.0129 |
| AUEC0–24 (%·h) | 2234 (18) | 1888 (32) | 346 (38–653) | 0.0302 |
| Collagen-induced | ||||
| IPAmax (%) | 83.0 (16) | 74.1 (18.4) | 8.7 (3.9–13.6) | 0.0017 |
| TIPAmax (h) | 4.0 (2–24) | 4.0 (2–12) | NA | NA |
| AUEC0–12 (%·h) | 939 (18) | 836 (19) | 103 (45–161) | 0.0019 |
| AUEC0–24 (%·h) | 1785 (18) | 1566 (20) | 219 (92–346) | 0.0023 |
Data are mean (coefficient of variation %) for IPAmax and AUEC; median (range) for TIPAmax. ADP = adenosine diphosphate; AUEC0–12, AUEC0–24 area under the effect curve of IPA from 0–12 hours and 0–24 hours, respectively; CI = confidence interval; IPA = inhibition of platelet aggregation; IPAmax = maximum IPA; TIPAmax = time to IPAmax. p-values are for ticagrelor/aspirin comparison to clopidogrel/aspirin dosing using ANOVA.
200 mg bid for 5 days then a single 200 mg dose for one day.
300 mg for one day then 75 mg qd for 5 days.
Mean IPAmax was significantly higher with ticagrelor/aspirin vs. clopidogrel/aspirin (p = 0.0077; Table II). With ticagrelor/aspirin, 100% IPAmax was achieved in 14/16 volunteers and was >94% in 2/16 volunteers. In contrast, with clopidogrel/aspirin the final IPAmax was >80% in 7/16 volunteers, 30–80% in 6/16 volunteers and <30% in 3/16 volunteers. AUEC0–12 and AUEC0–24 were also significantly higher with ticagrelor/aspirin vs. clopidogrel/aspirin (Table II).
Collagen-induced platelet aggregation
Study A: Effect of aspirin and ticagrelor
At 2 hours post-dosing with 300 mg aspirin alone, the mean (standard error of the mean [SEM]) final-extent IPA was 37% (± 4.2%). With ticagrelor alone, the mean final-extent IPA ranged from 7–9% (4–12 hours post-dose) and 12–20% (2–12 hours post-dose) with 50 mg bid and 200 mg bid ticagrelor, respectively (Figure 5a).
Figure 5.
Inhibition of platelet aggregation (final-extent, collagen-induced) following the administration of (a) ticagrelor at 50 mg bid for 5 days, and 200 mg bid for 4 days then qd for 1 day, ± once-daily aspirin (300 mg) (Study A), and (b) ticagrelor (200 mg bid for 5 days then a single 200 mg dose for 1 day) + aspirin (75 mg qd) and clopidogrel (loading dose 300 mg on Day 1 then 75 mg qd for 5 days) + aspirin (75 mg qd) (Study B). Values are mean ± SD.
The range of mean IPA was 66–74% (4–12 hours post-dose) and 72–76% (2–12 hours post-dose) with 50 mg bid and 200 mg bid ticagrelor co-administered with aspirin (300 mg) once-daily, respectively (Figure 5a).
Study B: Effect of aspirin and ticagrelor
At 2 hours post-dosing with 75 mg aspirin alone, the mean (SEM) final-extent IPA was 38% (± 3.6%). For ticagrelor (200 mg bid) co-administered with 75 mg aspirin daily, the mean final-extent IPA (collagen-induced) ranged from 74% to 82% at 2–12 hours post-dosing (Figure 5b).
Study B: Ticagrelor/aspirin vs. clopidogrel/aspirin
For collagen-induced platelet aggregation, mean final-extent IPA was higher with ticagrelor/aspirin vs. clopidogrel/aspirin at all time points evaluated (Figure 5b). IPAmax, AUEC0–12, and AUEC0–24 were also significantly higher with ticagrelor/aspirin vs. clopidogrel/aspirin (Table II).
Bleeding times
Study A: Effect of ticagrelor with and without aspirin (Simplate method)
At steady state, both doses of ticagrelor (no aspirin) increased bleeding time compared with baseline (Table III). Co-administration of aspirin (300 mg qd) with either dose of ticagrelor had no effect on bleeding times compared to ticagrelor alone (Table III). Following the administration of 200 mg bid ticagrelor with or without aspirin, most volunteers had bleeding times >30 minutes (1800 seconds).
Table III. .
Mean bleeding times following multiple dosing of ticagrelor ± once-daily aspirin and clopidogrel with once-daily aspirin.
| Method/treatment | Bleeding time, mean ± SD (range)† |
|---|---|
| Study A (n = 13–16)a | |
| Simplate method | |
| Baseline (at enrolment) | 274 ± 64 (120–390) |
| Ticagrelor (50 mg bidb) | 1376 ± 527 (540–1800) |
| Ticagrelor/aspirin (50 mg bidb/300 mg qd) | 1622 ± 377 (720–1800) |
| Ticagrelor (200 mg bid§) | 1800 ± 0 (1800–1800) |
| Ticagrelor/aspirin (200 mg bid§/300 mg qd) | 1800 ± 0 (1800–1800) |
| Lancet method | |
| Baseline (at enrolment) | 159 ± 54 (53–240) |
| Ticagrelor (200 mg bid§) | 250 ± 77 (135–435) |
| Ticagrelor/aspirin (200 mg bid§/300 mg qd) | 541 ± 469 (195–1800) |
| Study B (n = 8–16) | |
| Lancet method | |
| Baseline (at screening) | 2.2 ± 0.7 (1.1–3.0) |
| Aspirin (300/75 mg qdc) | |
| 4 h post-dose on Day 3 | 2.7 ± 0.6 (2.0–3.8) |
| Pre-dose on Day 4 | 2.2 ± 0.4 (1.5–2.8) |
| Ticagrelor/aspirin (200 mg bidd/75 mg qd) | |
| Pre-dose | 13.1 ± 10.0 (2.5–30.0) |
| 4 h post-dose | 8.7 ± 8.4 (2.3–30.0) |
| Clopidogrele/aspirin (75 mg qd/75 mg qd) | |
| Pre-dose | 4.4 ± 1.5 (2.0–8.1) |
| 4 h post-dose | 4.8 ± 1.9 (2.0–8.5) |
bid = twice daily; h = hours; qd = once daily; SD = standard deviation.
Time point = 4 hours post-dosing, except for baseline values;†bleeding times are in seconds for Study A, and minutes for Study B.
50 mg bid for 5 days; §200 mg bid for 4 days then a single 200 mg dose for one day.
Loading dose 300 mg on day 1 then 75 mg qd thereafter.
200 mg bid for 5 days then a single 200 mg dose for one day.
300 mg for one day then 75 mg qd for 5 days.
Study A: Effect of ticagrelor with and without aspirin (lancet method)
At the end of ticagrelor administration (50 mg bid for 5 days, followed by 200 mg bid for 4 days then 200 mg once) mean bleeding time at 4 hours post-dose was increased vs. baseline. Co-administration of aspirin (300 mg qd) further prolonged the bleeding time (Table III). Individual bleeding times >30 minutes were observed at four time points; all of these occurred with ticagrelor/aspirin treatment.
Study B: Effect of ticagrelor with and without aspirin (lancet method)
Aspirin alone (75 mg qd) did not increase bleeding time vs. baseline (Table III). Multiple (200 mg bid, 5 days) doses of ticagrelor co-administered with aspirin (75 mg qd) increased the bleeding time (Table III). However, bleeding time with ticagrelor/aspirin dosing did not appear to correlate with ticagrelor plasma concentrations; on Day 9 mean (standard deviation [SD]) bleeding time pre-dose was 13.1 (10.0) minutes and decreased at 4 hours post-dose to 8.7 (8.4) minutes. At the latter time point, plasma ticagrelor concentrations were two-fold higher than pre-dose values.
Study B: Comparative effects of ticagrelor/aspirin vs. clopidogrel/aspirin (lancet method)
Clopidogrel/aspirin (75 mg qd/75 mg qd for 5 days) administration prolonged the mean lancet bleeding time (Table III). At all time points evaluated, bleeding times were longer with ticagrelor/aspirin (200 mg bid/75 mg qd for 5 days) vs. clopidogrel/aspirin (Table III).
Tolerability
Overall, ticagrelor alone, ticagrelor with aspirin (75 mg qd and 300 mg qd), and clopidogrel/aspirin (75 mg qd/75 mg qd) were well tolerated. There were no serious AEs or deaths in either study.
In Study A, three volunteers discontinued treatment due to bleeding-related AEs; possibly ticagrelor-related. One volunteer had moderate epistaxis on Days 2 and 3 of ticagrelor (50 mg bid)/aspirin co-administration. Another volunteer discontinued due to hematoma (moderate), petechiae (mild), and urticaria (mild) commencing on Day 9 (ticagrelor 200 mg bid/aspirin). Moderate gingivitis, between Days 5 and 7 of ticagrelor 200 mg bid dosing, resulted in the discontinuation of another volunteer. Overall, nine volunteers had 14 AEs: four volunteers had four AEs with ticagrelor alone; six volunteers had 10 AEs with ticagrelor/aspirin; one volunteer had an AE in both periods. The most common AEs (>10% volunteers) were headache (n = 2 with ticagrelor) and petechiae (n = 2 with ticagrelor/aspirin).
In Study B, four volunteers had five AEs (mild). During ticagrelor/aspirin administration, three volunteers had one AE each (diarrhea; epistaxis; dental discomfort [not treatment related]). One volunteer had two AEs (nausea and flatulence) during treatment with clopidogrel/aspirin
In both studies, there were no clinically meaningful changes in hematology, clinical chemistry, urinalysis, vital signs, physical findings, or ECG data with ticagrelor, ticagrelor/aspirin, or clopidogrel/aspirin.
Discussion
The previously unpublished data presented here from two early studies in the ticagrelor development programme (2002–2003) indicate that aspirin is not associated with a pharmacokinetic or pharmacodynamic (IPA) effect on ticagrelor. Our current findings on IPA (ADP-induced) by ticagrelor are similar to those shown in a previous report [21]. Also in line with previous data [23], aspirin alone had minimal inhibitory effect on ADP-induced platelet aggregation (approximately 10%; Study B). In addition, the degree of IPA (ADP-induced) by ticagrelor was unaffected by aspirin (300 mg qd, Study A), and near-maximal IPA was achieved with ticagrelor (200 mg bid)/75 mg qd aspirin (Study B). These findings showing minimal effects of aspirin on ticagrelor IPA (ADP-induced) are unsurprising since the mechanism of action for IPA differs between these compounds. Aspirin blocks the cyclooxygenase activity thereby inhibiting arachidonic acid-induced platelet aggregation [12], whereas ticagrelor is a P2Y12 receptor antagonist and inhibits ADP-induced platelet aggregation [1].
Collagen was also used as an agonist of platelet aggregation in the present studies. Aspirin alone inhibited collagen-induced platelet aggregation by a magnitude similar to that observed previously in healthy volunteers (e.g. 37% IPA in males with 81 and 325 mg aspirin qd for 14 days) [24]. Ticagrelor alone at steady state slightly inhibited collagen-induced platelet aggregation in Study A. However, ticagrelor had a synergistic effect with the IPA associated with aspirin alone, since the IPA observed after ticagrelor (50 or 200 mg bid)/aspirin (300 mg qd) was higher than either drug administered alone. Moreover, IPA was similar after ticagrelor 200 mg bid/aspirin 300 mg qd (Study A) and ticagrelor 200 mg bid/aspirin 75 mg qd (Study B).
Pharmacokinetic data from the two present studies also demonstrate a lack of interaction between ticagrelor and aspirin. The steady-state pharmacokinetic parameters of ticagrelor and AR-C124910XX following the administration of ticagrelor alone (Study A) were consistent with previously reported data [21]. Co-administration of aspirin (300 mg qd; Study A) with both ticagrelor doses did not affect the pharmacokinetic parameters of either the parent compound or its major metabolite. In addition, the pharmacokinetic data with 200 mg bid ticagrelor/300 mg qd aspirin (Study A) were similar to those with 200 mg bid ticagrelor/75 mg qd aspirin (Study B). Our findings with ticagrelor plus aspirin in healthy volunteers are broadly consistent with steady-state pharmacokinetic parameters of ticagrelor and AR-C124910XX in the Phase II Dose confIrmation Study assessing anti-Platelet Effects of AZD6140 vs. clopidogRel in non-ST segment Elevation myocardial infarction (DISPERSE) trials, in which ticagrelor was co-administered with aspirin (75 mg qd) to patients with atherosclerosis [11] or ACS [10], [25]. Collectively, these findings demonstrate that aspirin co-administration at 75 or 300 mg qd did not affect exposure to ticagrelor or AR-C124910XX.
The therapeutic regimen of clopidogrel/aspirin was included in one of our studies (Study B) for comparative purposes. In this study, ADP-induced IPA with ticagrelor (200 mg bid)/aspirin (75 mg qd) was more rapid, reached higher levels, and was less variable compared with clopidogrel/aspirin. A greater level of collagen-induced IPA was also achieved with ticagrelor/aspirin than clopidogrel/aspirin. The current observations of higher ticagrelor IPA activity vs. clopidogrel confirm previously reported results in healthy volunteers (no aspirin [21]), and in patients (with aspirin [10], [11]).
Bleeding times in both studies highlighted several issues, including methodology factors. In Study A, data suggest that the lancet method was more sensitive than the Simplate method for detecting an increase in bleeding time. The two methods indicated an increase in bleeding time vs. baseline for ticagrelor of different magnitudes, and, in contrast to the lancet method, the Simplate method did not detect an increase in bleeding time on co-administration with aspirin (300 mg qd). However, in Study B, lancet bleeding times did not correlate with aspirin dose or bleeding-related AEs. Overall, these results demonstrate the unreliability of bleeding tests in predicting bleeding risk.
Ticagrelor alone or with aspirin was well tolerated at the studied doses, consistent with previously published studies in healthy volunteers [9], [21], [26], or in patients with atherosclerosis [11], or ACS [25]. PLATO data have also shown that the long-term administration of ticagrelor (90 mg bid) and aspirin (various dose levels) was well tolerated with no increase in overall rates of major bleeding vs. clopidogrel/aspirin [2].
A prespecified subgroup analysis of the PLATO trial demonstrated a significant interaction between treatment and region (p = 0.045), with attenuated benefit associated with ticagrelor being observed in North America compared with the rest of the world [2]. Two subsequent analyses have noted that play of chance cannot be ruled out as the reason for the interaction, and the regional difference may be explained by an underlying statistical interaction with aspirin maintenance dose [19].
A post-hoc subgroup analysis of the PLATO trial (Cox regression with median maintenance dose) showed that, in patients taking ≤100 mg maintenance aspirin, ticagrelor was associated with superior outcomes compared with clopidogrel (adjusted HR 0.77 [95% CI, 0.69−0.86]. In patients taking maintenance aspirin dose ≥300 mg, HR of 1.45 (95% CI, 1.01–2.09) favored clopidogrel. The interaction between aspirin dose category and treatment is significant (p = 0.00006) [19]. Subsequently, the FDA recommended that ticagrelor be co-administered with 75–100 mg qd aspirin [3].
The mechanism of the antagonism between high-dose aspirin and ticagrelor on clinical outcomes is currently under investigation, a detailed discussion of which is beyond the scope of this manuscript. In brief, it is thought that the antagonism of P2Y12 receptors, such as that seen with ticagrelor, may also inhibit thromboxane-induced platelet activation [27], [28] in addition to ADP-dependent platelet activation. Consequently, aspirin-induced blockade of thromboxane production, which reaches maximum effect at relatively low doses, will be unable to provide additional IPA [27], [28].
The present studies were performed in healthy volunteers who had no evident coronary artery disease. In addition, the doses of ticagrelor and aspirin employed in these two studies differed from those used in the PLATO trial, i.e. ticagrelor 180 mg loading dose followed by 90 mg bid [2]. Aspirin maintenance doses of 75–100 mg daily were recommended in the PLATO study, with 325 mg daily aspirin recommended in patients who received a stent, but as previously noted, higher aspirin doses were used in the USA [19]. However, aside from these study limitations, these data suggest that the effects of aspirin on the pharmacokinetics or pharmacodynamics (IPA) of ticagrelor are unlikely to be responsible for differences observed in the PLATO trial.
Conclusions
Ticagrelor pharmacokinetics following the administration of 50 mg bid and 200 mg bid ticagrelor doses were unaffected by the co-administration of 300 mg qd aspirin. This concomitant dose of aspirin also had no effect on ticagrelor-induced IPA. Ticagrelor/aspirin co-administration was well tolerated at all dose combinations evaluated.
Acknowledgments
The authors wish to thank Dr Thierry Duvauchelle and his team for conducting both studies at ASTER, Paris, France. Staff at York Bioanalytical Solutions, York, UK, are acknowledged for their pharmacokinetic analyses. We also thank Jackie Phillipson (Gardiner-Caldwell Communications), who provided medical writing support funded by AstraZeneca.
Declaration of interest: Drs Butler, Maya, and Teng are employees of AstraZeneca.
References
- 1.Husted S, van Giezen JJ. Ticagrelor: The first reversibly binding oral P2Y receptor antagonist. Cardiovasc Ther. 2009;27:259–274. doi: 10.1111/j.1755-5922.2009.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wallentin L, Becker RC, Budaj A, Cannon CP, Emanuelsson H, Held C, Horrow J, Husted S, James S, Katus H et al. for the PLATO investigators. Ticagrelor vs. clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361:1045–1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 3.Brilinta TM US. full prescribing information, July 2011. [Accessed 18 July 2012] Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/022433s000lbl.pdf. [Google Scholar]
- 4. Brilique, summary of product characteristics, 2010. [Accessed 18 July 2012]. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001241/WC500100494.pdf.
- 5.Guyatt GH, Akl EA, Crowther M, Gutterman DD, Schuünemann HJ. Executive summary: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College fo Chest Physicians evidence-based clinical practice guidelines. Chest. 2012;141:7S–47S. doi: 10.1378/chest.1412S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hamm CW, Bassand JP, Agewall S, Bax J, Boersma E, Bueno H, Caso P, Dudek D, Gielen S, Huber K et al. ESC guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur Heart J. 2011;32:2999–3054. doi: 10.1093/eurheartj/ehr236. [DOI] [PubMed] [Google Scholar]
- 7.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, Chambers CE, Ellis SG, Guyton RA, Hollenberg SM et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J Am Coll Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Teng R, Oliver S, Hayes M, Butler K. Absorption, distribution, metabolism, and excretion of ticagrelor in healthy subjects. Drug Metab Dispos. 2010;38:1514–1521. doi: 10.1124/dmd.110.032250. [DOI] [PubMed] [Google Scholar]
- 9.Teng R, Butler K. Pharmacokinetics, pharmacodynamics, tolerability, and safety of single ascending doses of ticagrelor: A reversibly binding oral P2Y12 receptor antagonist in healthy subjects. Eur J Clin Pharmacol. 2010;66:487–496. doi: 10.1007/s00228-009-0778-5. [DOI] [PubMed] [Google Scholar]
- 10.Storey RF, Husted S, Harrington RA, Heptinstall S, Wilcox RG, Peters G, Wickens M, Emanuelsson H, Gurbel P, Grande P et al. Inhibition of platelet aggregation by AZD6140, a reversible oral P2Y12 receptor antagonist, compared with clopidogrel in patients with acute coronary syndromes. J Am Coll Cardiol. 2007;50:1852–1856. doi: 10.1016/j.jacc.2007.07.058. [DOI] [PubMed] [Google Scholar]
- 11.Husted S, Emanuelsson H, Heptinstall S, Sandset PM, Wickens M, Peters G. Pharmacodynamics, pharmacokinetics, and safety of the oral reversible P2Y12 antagonise AZD6140 with aspirin in patients with atherosclerosis: A double-blind comparison to clopidogrel with aspirin. Eur Heart J. 2006;27:1038–1047. doi: 10.1093/eurheartj/ehi754. [DOI] [PubMed] [Google Scholar]
- 12.Wu KK. Aspirin and other cyclooxygenase inhibitors: New therapeutic insights. Semin Vasc Med. 2003;3:107–112. doi: 10.1055/s-2003-40668. [DOI] [PubMed] [Google Scholar]
- 13.Patrignani P, Filabozzi P, Patrono C. Selective cumulative inhibition of platelet thromboxane production by low-dose aspirin in healthy subjects. J Clin Invest. 1982;69:1366–1372. doi: 10.1172/JCI110576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infraction, and stroke in high risk patients. Br Med J. 2002;324:71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Antithrombotic Trialists' Collaboration Aspirin in the primary and secondary prevention of vascular disease: Collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373:1849–1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patrono C, Coller B, Dalen JE, FitzGerald GA, Fuster V, Gent M, Hirsh J, Roth G. Platelet-active drugs: The relationships among dose, effectiveness and side effects. Chest. 2001;119:39S–63S. doi: 10.1378/chest.119.1_suppl.39s. [DOI] [PubMed] [Google Scholar]
- 17.Campbell CL, Smyth S, Montalescot G, Steinhubl SR. Aspirin dose for the prevention of cardiovascular disease: A systematic review. JAMA. 2007;297:2018–2024. doi: 10.1001/jama.297.18.2018. [DOI] [PubMed] [Google Scholar]
- 18.Berger JS, Sallum RH, Katona B, Maya J, Ranganathan G, Xu Y, Mwamburi M. Is there an association between aspirin dosing and cardiac and bleeding events after treatment of acute coronary syndrome? A systematic review of the literature. Am Heart J. 2012;164:153–162.e5. doi: 10.1016/j.ahj.2012.04.001. [DOI] [PubMed] [Google Scholar]
- 19.Mahaffey KW, Wojdyla DM, Carroll K, Becker RC, Storey RF, Angiolillo DJ, Held C, Cannon CP, James S, Pieper KS et al. on behalf of the PLATO investigators. Ticagrelor compared with clopidogrel by geographic region in the platelet inhibition and patient outcomes (PLATO) trial. Circulation. 2011;124:544–554. doi: 10.1161/CIRCULATIONAHA.111.047498. [DOI] [PubMed] [Google Scholar]
- 20.Sillén H, Cook M, Davis P. Determination of ticagrelor and two metabolites in plasma samples by liquid chromatography and mass spectrometry. J Chromatogr B. 2010;878:2299–2306. doi: 10.1016/j.jchromb.2010.06.018. [DOI] [PubMed] [Google Scholar]
- 21.Butler K, Teng R. Pharmacokinetics, pharmacodynamics and safety and tolerability of multiple ascending doses of ticagrelor in healthy volunteers. Br J Clin Pharmacol. 2010;70:65–77. doi: 10.1111/j.1365-2125.2010.03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Šrámek R, Šrámek A, Koster T, Briet E, Rosendaal FR. A randomized and blinded comparison of three bleeding time techniques: The Ivy method, and the Simplate II® method in two directions. Thromb Haemost. 1992;67:514–518. [PubMed] [Google Scholar]
- 23.Penz SM, Bernlochner I, Tóth O, Lorenz R, Calatzis A, Siess W. Selective and rapid monitoring of dual platelet inhibition by aspirin and P2Y12 antagonists by using multiple electrode aggregometry. Thromb J. 2010;8:9. doi: 10.1186/1477-9560-8-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qayyum R, Becker DM, Yanek LR, Moy TF, Becker LC, Faraday N, Vaidya D. Platelet inhibition by 81 and 325 mg aspirin daily in men vs. women without clinically apparent cardiovascular disease. Am J Cardiol. 2008;101:1359–1363. doi: 10.1016/j.amjcard.2007.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cannon CP, Husted S, Harrington RA, Scirica BM, Emanuelsson H, Peters G, Storey RF. DISPERSE-2 Investigators. Safety, tolerability, and initial efficacy of AZD6140, the first reversible oral adenosine diphosphate receptor antagonist, compared with clopidogrel, in patients with non-ST-segment elevation acute coronary syndrome: Primary results of the DISPERSE-2 Trial. J Am Coll Cardiol. 2007;50:1844–1851. doi: 10.1016/j.jacc.2007.07.053. [DOI] [PubMed] [Google Scholar]
- 26.Teng R, Butler K. AZD6140, the first reversible oral platelet P2Y12 receptor antagonist, has linear pharmacokinetics and provides near complete inhibition of platelet aggregation, with reversibility of effect in healthy subjects. Can J Clin Pharmacol. 2008;15:e426. [Google Scholar]
- 27.Armstrong PCJ, Leadbeater PD, Chan MV, Kirkby NS, Jakubowski JA, Mitchell JA, Warner TD. In the presence of strong P2Y12 receptor blockade, aspirin provides little additional inhibition of platelet aggregation. J Thromb Haemost. 2011;9:552–561. doi: 10.1111/j.1538-7836.2010.04160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kirkby NS, Leadbeater PDM, Chan MV, Nylander S, Mitchell JA, Warner TD. Anti-platelet effects of aspirin vary with level of P2Y12 receptor blockade supplied by either ticagrelor or prasugrel. J Thromb Haem. 2011;9:2103–2105. doi: 10.1111/j.1538-7836.2011.04453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]