Figure 1.
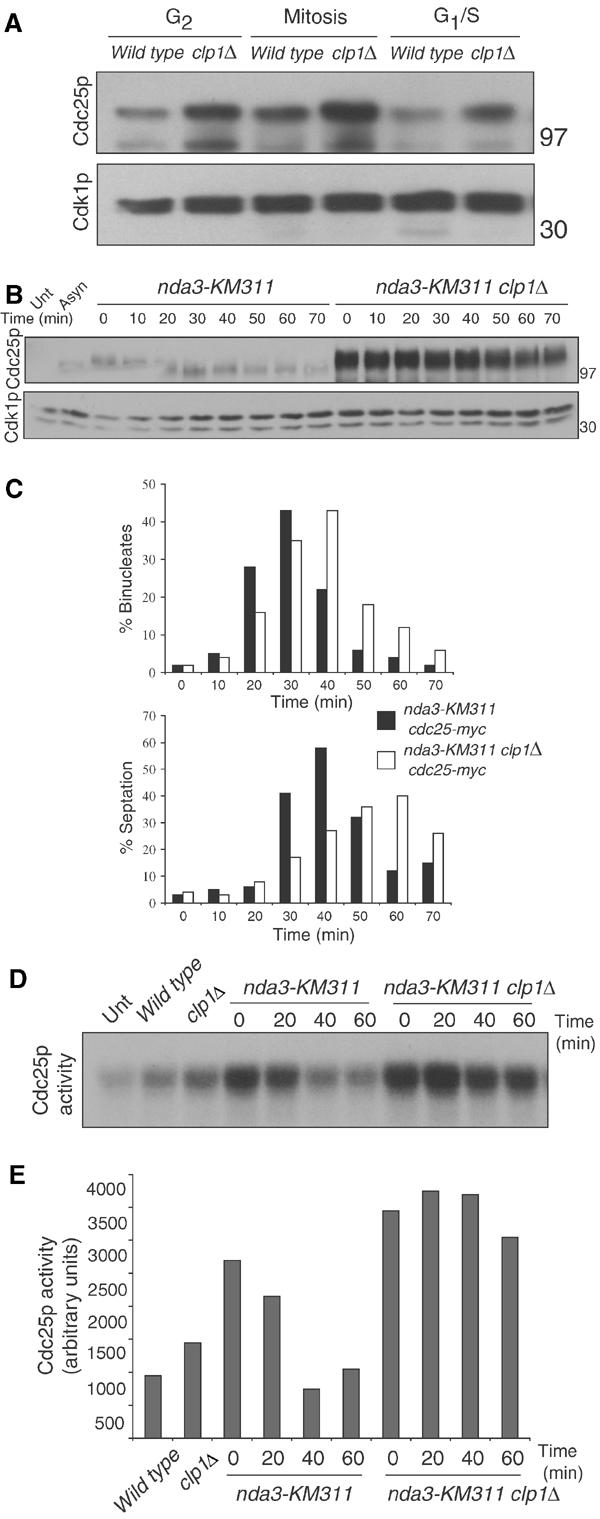
clp1+ regulation of Cdc25p. (A) cdc25-myc (KGY 3377) or cdc25-myc clp1Δ (KGY 3499) were grown to mid-log phase and synchronized by centrifugal elutriation. Samples were taken after synchronization and processed for protein (A) and cell cycle stage by DAPI staining and cell measurement analysis as follows: G2 cells were uninucleate and 8–12 μm in length, mitotic cells were uninucleate or binucleate cells with condensed chromatin, and G1/S cells contained two interphase nuclei and a septum (data not shown). Cdc25p-myc and Cdk1p (serves as loading control in each of our blots) were detected by immunoblotting with 9E10 and anti-PSTAIR antibodies, respectively. Numbers adjacent to the blots indicate the position of molecular weight standards on this and all subsequent blots. (B) nda3-KM311 cdc25-myc (KGY 3916) and nda3-KM311 cdc25-myc clp1Δ (KGY 4213) were grown to mid-log phase and synchronized by cold shift to 18°C for 7 h. Cells were then released to the permissive temperature (32°C), and samples were collected at the indicated time points. Extracts were prepared from these and processed for immunoblot analysis. (C) Completion of mitotic exit was monitored by determining binucleate formation (top panel) and septation index (lower panel). For the Cdc25p immunoblot, samples were immunoprecipitated with 9E10 antibody and immunoblotted with anti-Cdc25p antibodies. (D) Cells were synchronized as in (B) and samples were collected at the indicated time points. Extracts were prepared and immunoprecipitated with 9E10 antibody. Samples were processed for Cdc25p activity and quantified and normalized to basal levels of activity (Unt) in (E) indirectly via activation of Cdk1p's histone H1 activity as described in Materials and methods. In this experiment, Cdc25p activity was a saturable reaction (data not shown).
