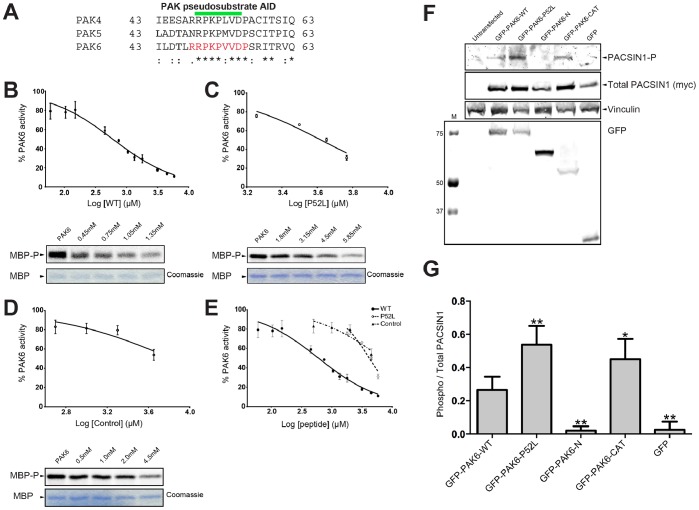
Figure 2. P52L mutation results in loss of PAK6 autoinhibition.
A) Alignment of the pseudosubstrate autoinhibitory domain (AID) of the type II PAK family members is shown. Pseudosubstrate region indicated by a green bar. Peptide used in panel B indicated in red. Conservation shown with “*” indicating identical, “:” indicating highly conserved and “.” Indicating semi-conserved. B–D) Dose-dependent relationship of the inhibition of wild-type (RRPKPVVDP), P52L (RRPKLVVDP) mutant, and control (APRTPGGRR) peptides on PAK6 kinase activity. B) Inhibition of PAK6 by wild-type (WT) peptide shown on a log scale. Data shows a mean and S.E.M error bar with 3 independent experiments. Concentration range from 0.06 mM to 5.85 mM of peptides was tested. C) Inhibition of PAK6 by P52L peptide shown on a log scale. Data shows a mean and S.E.M error bar with 3 independent experiments. Concentration range from 1.8 mM to 5.85 mM of peptides was tested. D) Inhibition of PAK6 by a control peptide. Data show a mean and S.E.M error bar with 3 independent experiments. Concentration range from 0.5 mM to 4.5 mM of peptide was tested. E) Inhibition plots from panels B-D shown on the same graph. F) Co-transfection of PAK6 with PACSIN1. Co-transfection of myc-PACSIN1 and GFP-PAK6 constructs probed for PACSIN1 phosphorylation using a phospho-specific PACSIN1 antibody. M indicates molecular weight marker. G) Quantification of E. Ratio of phosphorylated PACSIN1 to total PACSIN1 expressed is shown, normalized for Phospho PACSIN1/Total PACSIN1 (myc). PACSIN1 shows significantly increased phosphorylation when co-transfected with the P52L mutant compared to the wild-type PAK6 (* indicates p<0.05; ** indicates p<0.01; Student’s t-test). n = 4. Error bars indicate S.E.M.