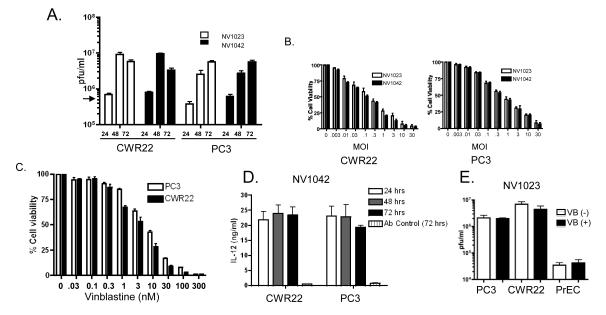
Figure 2.
Analysis of NV1023 and NV1042 replication and effects on prostate cancer cell killing (A) Single burst assays were performed using either NV1023 or NV1042 (MOI =1.5) on CWR22 or PC3 cells over a 72 hr period. Virus (cell pellet plus supernatants) on days 1, 2 and 3 post-infection and viral titers (pfu/ml) determined by plaque assay on Vero cells as described previously13. Input virus: ~4×105 plaque forming units (pfu; indicated by arrow). (B) Cell susceptibility assays were performed using NV1023 or NV1042 on either CWR22 (left) or PC3 (right) prostate cancer cells. Cell lines were inoculated with the indicated MOI’s of virus and cell killing was evaluated by MTS assay on day 3 (error bars represent S.E.M.). (C) PC3 (open bar) or CWR22 (solid bar) prostate cancer cells were treated with increasing concentration of VB (0.01-100 nM) and MTS assays were performed to generate dose-response curves. (D) Supernatants collected from single burst assays were assayed for IL-12 derived from NV1042 by ELISA at the indicated time points. NV1023 was used as a control vector to normalize for background andnormal goat IgG control was also included to demonstrate specificity for murine IL-12. (E) Prostate cancer (PC3 and CWR22) and normal prostate epithelial (PrEC) cells were incubated in the presence or absence of non-toxic concentrations of VB (0.1 nM) for 12h and thereafter, cells were infected with NV1023 (MOI of 1.5) for 72 hrs. Virus titers were determined by plaque assay on Vero cells. Note that virus replication is not altered in the presence of VB and is negligible in PrEC.