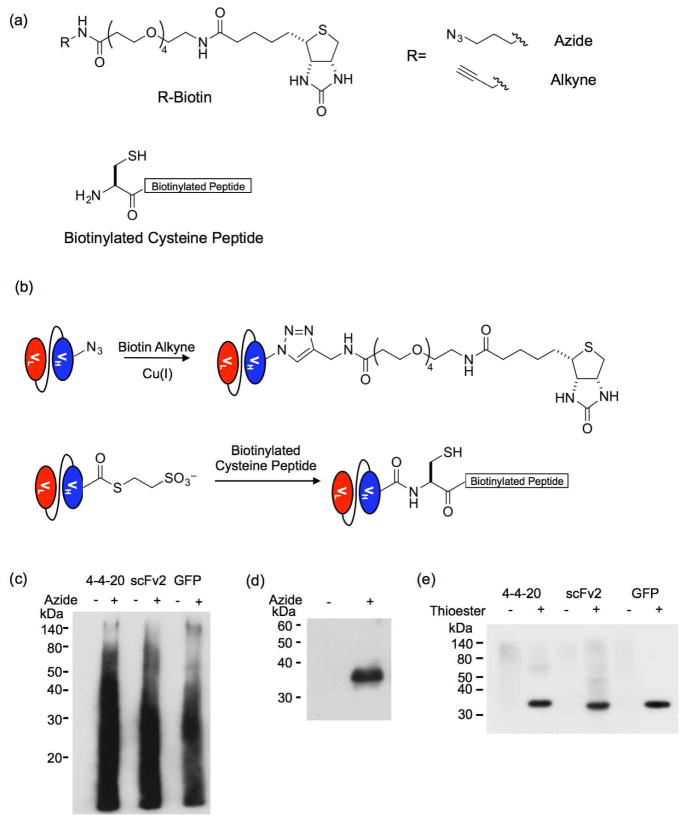
Figure 3.
Site-specific biotinylation of released proteins. (a) Structures of the biotinylation reagents, biotin alkyne, biotin azide and cysteine-terminated biotinylated peptide. (b) The biotin alkyne reacts with the azide-functionalized proteins obtained via cleavage reaction with the hydrazine azide in a copper-catalyzed azide–alkyne cycloaddition (CuAAC) reaction. The protein thioester obtained by the cleavage reaction with MESNA reacts with a N-terminal cysteine peptide in an expressed protein ligation (EPL) reaction. (c) A Western blot of the CuAAC reaction probed with an anti-biotin antibody reveals that multiple proteins react with the biotin alkyne when the hydrazine azide is used to release the proteins from the yeast surface. When a non-azido hydrazine is used to cleave the proteins, no reaction with the biotin alkyne is detected. (d) The hydrazine-released products were subjected to FLAG tag purification before CuAAC reaction with biotin alkyne. A Western blot of the reaction probed an anti-biotin antibody reveals biotinylation corresponds to the hydrazine-azide released proteins (shown for GFP only). (e) An anti-biotin Western blot of the EPL reaction between the protein thioesters and biotinylated cysteine peptide shows specific biotinylation of the released protein. Hydrazine-released proteins were reacted with the biotinylated cysteine peptide as a non-thioester negative control to demonstrate that the reaction is specific to the thioester.