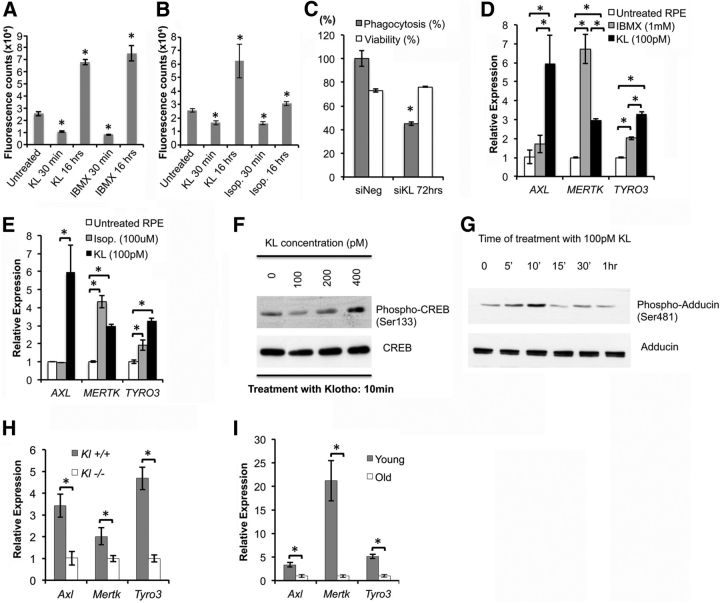
Figure 3.
KL regulates phagocytosis in human and mouse RPE cells by upregulating MERTK gene expression. A, B, Treatment of RPE cells with 10 ng/ml (100 pm) recombinant KL protein results in a short-term (30 min) decrease and a long-term (16 h) increase in phagocytosis efficiency. Treatment with 1 mm IBMX (A) or with 100 μm isoproterenol (B) for 1 h has a similar effect on the phagocytosis efficiency 30 min and 16 h later. Results are the average ± SD of three independent experiments. RPE in six wells of a 96-well culture dish were used in each group. Asterisks indicate statistically significant differences in phagocytosis efficiency compared with the untreated control, as determined by the t test (p < 0.05). p values in A: 7.8 × 10−5 (KL 30 min); 1.3 × 10−6 (KL 16 h); 4.0 × 10−5 (IBMX 30 min); 2.1 × 10−5 (IBMX 16 h). p values in B: 2.0 × 10−4 (KL 30 min); 8.0 × 10−4 (KL 16 h); 4.6 × 10−5 [isoproterenol (Isop.) 30 min]; 2.7 × 10−4 (Isop. 16 h). C, RNAi knockdown of KL gene in RPE cells significantly reduces phagocytosis efficiency 72 h post-transfection. *p = 2.3 × 10−4 compared with scrambled siRNA (siNeg) control. Results represent two independent experiments, with 6 wells of a 96-well plate used in each group. D, E, Expression of genes important in phagocytosis in RPE cells (i.e., AXL, MERTK, TYRO3) is significantly increased by a 16 h treatment of RPE with KL protein, explaining the long-term effect of KL on phagocytosis. Treatment with 1 mm IBMX (D) or with 100 μm isoproterenol (E) for 1 h could also induce the expression of MERTK and TYRO3, but not of AXL, 16 h later. Asterisks indicate statistically significant differences between the untreated RPE cells and the RPE cells treated with IBMX, isoproterenol, or KL in the gene expression of the indicated genes, as determined by the t test (p < 0.05). Specifically, only KL treatment was able to increase the expression of AXL (D, E) compared with untreated RPE cells (p = 0.02) and RPE cells treated with IBMX (p = 0.05, D) or isoproterenol (p = 0.03, E). MERTK expression increased with KL treatment (p = 0.001, D, E), as well as with IBMX (p = 0.006, D) and isoproterenol (p = 0.026, E) treatments. IBMX treatment resulted in ∼50% higher levels of MERTK compared with the KL treatment (p = 0.01, D). Similarly, TYRO3 gene expression was significantly increased by KL (p = 0.002, D, E), IBMX (p = 9 × 10−4, D), and by isoproterenol (p = 0.009, E). KL treatment resulted in ∼30% higher TYRO3 levels compared with the TYRO3 increase caused by the IBMX treatment (p = 0.01, D). F, KL protein induces CREB phosphorylation in a concentration-dependent manner. RPE cells were treated with increasing doses of KL (0–400 pm) for 10 min and then lysed for Western blot analysis. A PKA-dependent increase in phosphorylation of CREB at Ser133 was revealed at 400 pm KL with a phospho-specific antibody. Levels of total CREB protein were not altered. G, KL protein induces PKA-dependent phosphorylation of adducin (at Ser481). RPE cells were treated with 100 pm KL and then lysed in RIPA buffer at the indicated time points for Western blot analysis. Phospho-adducin levels were detected by a specific antibody. Total adducin protein levels are also shown for normalization. The increase in phosphorylation of adducin caused by KL in 5–10 min may explain the inhibitory effect on RPE phagocytosis observed with short-term (30 min) treatment of RPE with 100 pm KL in Figure 3, A and B. H, I. Gene expression of phagocytosis factors (Axl, Mertk, Tyro3) is significantly reduced in the Kl−/− mouse retinas (H) compared with the Kl+/+ mouse retinas (H), and in old mouse retinae (13 months, I), compared with young mouse retinae (3 months, I). p values in H: 0.035 (Axl), 0.034 (Mertk) and 0.004 (Tyro3). p values in I: 0.004 (Axl), 0.015 (Mertk), and 0.002 (Tyro3). For the qRT-PCR analysis in H, RNA samples were isolated from the retinae of three Kl+/+ and three Kl−/− mice, and were analyzed separately. For the qRT-PCR in I, the RNA samples were isolated from the retinae of six young (3 months old) and six old (13 months old) C57BL/6 mice, and also were analyzed separately. The RNA samples in D and E were normalized to human GAPDH; in H and I, the mouse β-actin (Actb) was used for normalization, and the relative expression levels for each gene were calculated with the ΔΔCt method. The qRT-PCR analyses (D, E, H, and I) were performed in triplicate, and the average ± SD is shown for each sample.