Abstract
Phytoextraction is gaining acceptance as a cost-effective and environmentally friendly phytoremediation strategy for reducing toxic metal levels from contaminated soils. Cognizant of the potential of this phytoremediation technique as an alternative to expensive engineering-based remediation technologies, experiments were conducted to evaluate the suitability of some plants as phytoextraction species. From one of our preliminary studies, we found that tall fescue (Festuca arundinacea Schreb. cv. Spirit) can tolerate and accumulate significant amounts of lead (Pb) in its shoots when grown in Pb-amended sand. To further evaluate the suitability of tall fescue as one of the potential crop rotation species for phytoextraction, a study was conducted to determine whether the addition of ethylenediaminetetraacetic acid (EDTA) alone or in combination with acetic acid can further enhance the shoot uptake of Pb. Seeds were planted in 3.8 L plastic pots containing top soil, peat, and sand (4:2:1, v:v:v) spiked with various levels (0,1000, 2000 mg Pb/kg dry soil) of lead. At six weeks after planting, aqueous solutions (0, 5 mmol/kg dry soil) of EDTA and acetic acid (5 mmol/kg dry soil) were applied to the root zone, and all plants were harvested a week later. Results revealed that tall fescue was relatively tolerant to moderate levels of Pb as shown by non-significant differences in root and shoot biomass among treatments. An exception to this trend however, was the slight reduction in root and shoot biomass of plants exposed to the highest Pb level in combination with the two chelates. Root Pb concentration increased with increasing level of soil-applied Pb. Further increases in root Pb concentrations were attributed to chelate amendments. Translocation index, which is a measure of the partitioning of the metal to the shoots, was significantly enhanced with chelate addition especially when both EDTA and acetic acid were used. Chelate-induced increases in translocation indices correspondingly led to higher shoot Pb concentrations.
Keywords: phytoextraction, lead, chelates, phytoremediation, tall fescue
Introduction
Metals are anthropogenically released into the environment at increasing rates by mining, industry, and agriculture, causing serious problems for environmental and human health [1–4]. In the USA alone, more than 50,000 metal-contaminated sites await remediation, many of them Superfund sites [5]. In spite of the ever-growing number of toxic metal-contaminated sites, the most commonly used methods dealing with heavy metal pollution are either the extremely costly process of excavation and burial or simply isolation of the contaminated sites. Such cleanup is practical only for small areas, often a hectare or less [6]. Recent U.S. remediation costs have been estimated at $7 billion to $8 billion per year, approximately 35% of which involves remediation of metals [7].
Recently, heavy metal phytoextraction has emerged as a promising, cost-effective alternative to the conventional engineering-based remediation [8–12]. The objective of phytoextraction is to reduce heavy metal levels below regulatory limits within a reasonable time frame [9]. To achieve this objective, plants must accumulate high levels of heavy metals and produce high amounts of biomass. Early phytoextraction research dealt with hyperaccumulating plants, which have the ability to concentrate high amounts of heavy metals in their tissues [10, 13]. However, hyperaccumulators often accumulate only a specific element and are slow-growing, low-biomass-producing plants with little known agronomic or horticultural attributes. Moreover, there is no known hyperaccumulating plant for Pb, one of the most widespread and toxic metal pollutants in soils.
Previous hydroponic studies revealed that uptake and translocation of heavy metals in plants are enhanced by increasing heavy metal concentration in the nutrient solution [14]. The bioavailability of heavy metals in the soil is therefore, of paramount importance for successful phytoextraction. Lead has limited solubility in soils, and its availability for plant uptake is minimal due to complexation with organic and inorganic soil colloids, sorption on oxides and clays, and precipitation as carbonates, hydroxides, and phosphates [15–16]. Therefore, successful phytoextraction must include mobilization of heavy metals into the soil solution that is in direct contact with the roots. In most soils capable of supporting plant growth, the readily available levels of heavy metals, especially Pb, are low and do not allow substantial plant uptake if chelates are not applied. Chelates have been shown to desorb heavy metals from the soil matrix into soil solution [17], facilitate metal transport into the xylem, and increase metal translocation from roots to shoots of several fast-growing, high-biomass-producing plants [18–27].
Using a Pb-amended sand [28], tall fescue (Festuca arundinacea Schreb. cv. Spirit) was identified as a potential phytoextraction species because of its high biomass yield under elevated Pb levels and its ability to translocate high amounts of Pb into its shoots. The main objective of this study was to further evaluate the effectiveness of tall fescue as a phytoextraction species. We envisioned that this species can be used in a crop rotation scheme during the colder months, and also serves as cover crop for an otherwise barren metal-contaminated soil. Specifically, this experiment was conducted to determine whether pre-harvest amendments of ethylenediaminetetraacetic acid (EDTA) alone or in combination with acetic acid can further enhance the shoot accumulation (i.e., translocation index) of Pb by tall fescue grown on a Pb-contaminated soil.
Materials and Methods
Plant Culture and Experimental Design
Plants were maintained under a naturally-lit greenhouse with 31°C/20°C day/night temperatures. Supplemental light for 12 hours were provided by high intensity super halide lamps (1000 W H.Y. Lites Horizontal System, High Yield, Inc., Camas, WA). The photosynthetically active radiation (PAR; 400–700 nm) measured at the canopy level was no less than 1400 μmol photons m−2 sec−1 as measured with a LI-COR 6200 portable photosynthesis system (LI-COR, Inc., Lincoln, NE). Tall fescue (Festuca arundinacea Schreb. cv. Spirit) seeds were obtained from Hutto’s Garden, Jackson, MS. Unless otherwise specified, six seeds were sown in each 3.8 L plastic pot containing a growth medium composed of sieved silty clay loam soil (pH 8.2; 1.5% organic matter), peat, and sand mixed in 4:2:1 volumetric proportions. Emerged seedlings were thinned out to 5 plants per pot at 5 days after planting. Using a hand trowel, three concentrations (0, 1000, 2000 mg Pb/kg dry soil) of lead (supplied as lead nitrate) were thoroughly mixed with the soil. The Pb levels used are within previously reported Pb concentration ranges found in various contaminated sites [12, 18, 19]. The Pb-spiked soils with moisture contents maintained at field capacity were then allowed to equilibrate inside the greenhouse for 21 days prior to planting. Using previously described sequential extraction procedures [29], this Pb-spiked soil had the following percentages of Pb distributed in the various soil fractions: exchangeable (34.5%), carbonates (43.5%), Fe-Mn oxides (11.5%), organic matter (4.3%), residual (6.2%). Plants were watered every 2 to 3 days, depending on the evaporative demand, with full strength nutrient solution [30–31]. EDTA (0 or 5 mmol/kg dry soil) was applied as a 100 mL aqueous solution one week before harvest (pre-harvest). Moreover, a 100 mL aqueous solution of acetic acid (5 mmol/kg dry soil) was also added to some of the treatments one week before harvest. On average, 100 mL of nutrient solution were added to each pot to ensure that soil moisture content was maintained at field capacity and that no excess soil moisture drained from perforations at the bottom of each pot. A 17.8-cm plastic saucer was placed beneath each pot to prevent cross contamination among treatments.
Any symptoms of metal toxicity (e.g., discoloration, pigmentation, yellowing, necrosis, stunting) exhibited by plants were visually noted during the experimental period. All plants were harvested at seven weeks after planting. For dry biomass determinations, shoots and roots were separated during harvest then oven-dried at 70°C for 48 hours. Prior to oven drying, roots were washed with distilled water to remove any adhering debris.
Metal Extraction and Analyses
Dried samples were weighed and ground in a Wiley mill equipped with a 425 μm (40-mesh) screen. Lead contents of each 200 mg dry, ground plant tissue were extracted using previously described procedures [32] with slight modifications [30–31]. Briefly, 40 ml of 50% aqueous nitric acid were added to a 250 ml Erlenmayer flask containing a representative sample of ground tissue. The acidified sample was heated to 95 °C, refluxed for 15 minutes without boiling and then allowed to cool. Another 10 ml of 50 % aqueous nitric acid were added and the sample was again heated and refluxed for 30 minutes. The heated sample was allowed to cool, then completely oxidized in 5 ml concentrated nitric acid. The oxidized solution was allowed to evaporate to approximately 5ml without boiling. To initiate the peroxide reaction, 2 ml of deionized distilled water and 3ml of 30% hydrogen peroxide were added to the concentrated digestate and then heated until effervescence subsided. Another 7 ml of 30% hydrogen peroxide were added continuously in 1 ml aliquots as the digestate was again heated. The digestate was heated until effervescence was minimal and its volume reduced to approximately 5 ml. After cooling, the final digestate was diluted to about 100 ml with deionized, distilled water. The digestate was filtered through a filter paper (Whatman No. 1) and the final volume was adjusted to 100 ml with deionized, distilled water.
Lead concentrations were quantified using atomic absorption spectrometry (Thermo Jarrell Ash Model AA Scan 4) and expressed as mg Pb/kg dry weight of plant tissue. This analytical system had a 98% recovery efficiency and detection limit of 5 parts per billion (ppb) Pb. Per cent translocation index (T.I.) was calculated using the formula described previously by Athalye et al. [33]: T.I. = (shoot Pb accumulation) × 100/ (shoot + root Pb accumulation).
Statistical Analysis
In this experiment, each treatment replicate consisted of one pot containing 5 plants. Treatments were arranged in a completely randomized design (CRD) with four replications. Data were analyzed using Statistical Analysis System (SAS). Treatment comparisons were done using Fisher’s Protected Least Significant Difference (LSD) test. In this study, a probability p≤0.05 was considered to be statistically significant.
Results
The Pb treatments alone did not significantly affect root biomass of tall fascue plants (Table 1). However, root biomass of plants grown at 1000 and 2000 mg Pb/kg were reduced by 24% and 28%, respectively with the addition of EDTA alone, and in combination with acetic acid. Both Pb and chelate amendments did not affect shoot biomass (Table 1). This observation was also supported by the absence of any discernible phytotoxic symptoms (e.g., stunting, chlorosis, necrosis, discoloration, pigmentation) exhibited by the shoots.
Table 1.
Effects of various concentrations of Pb and chelates on root and shoot dry biomass of tall fescue. For each organ, means with a similar letter do not differ significantly using Fisher’s Protected LSD test (p ≤0.05).
| Treatment Lead (mg Pb/kg) | EDTA (mmol/kg) | Biomass (mg/plant) | |
|---|---|---|---|
|
| |||
| Root±SEM | Shoot±SEM | ||
| 0 | 0 | 130.3ab ±4.8 | 269.8a ± 15.7 |
| 0 | 5 Pre-harvest | 132.8a ± 8.4 | 245.8ab ± 13.3 |
| 1000 | 0 | 121.8a ± 2.5 | 249.8ab ± 18.2 |
| 1000 | 5 Pre-harvest | 105.0c ± 12.2 | 236.8ab ± 8.4 |
| 1000 | 5* Pre-harvest | 100.5c ± 8.5 | 243.8ab ± 12.2 |
| 2000 | 0 | 116.0abc ± 6.4 | 228.8b ± 13.3 |
| 2000 | 5 Pre-harvest | 109.3bc ± 9.6 | 219.8b ± 14.7 |
| 2000 | 5 *Pre-harvest | 107.3c ± 4.8 | 225.5b ± 6.7 |
indicates that an aqueous solution of acetic acid (5 mmol/kg dry soil) was added at the same time as the aqueous solution of EDTA; (SEM= standard error of the mean of 4 replications).
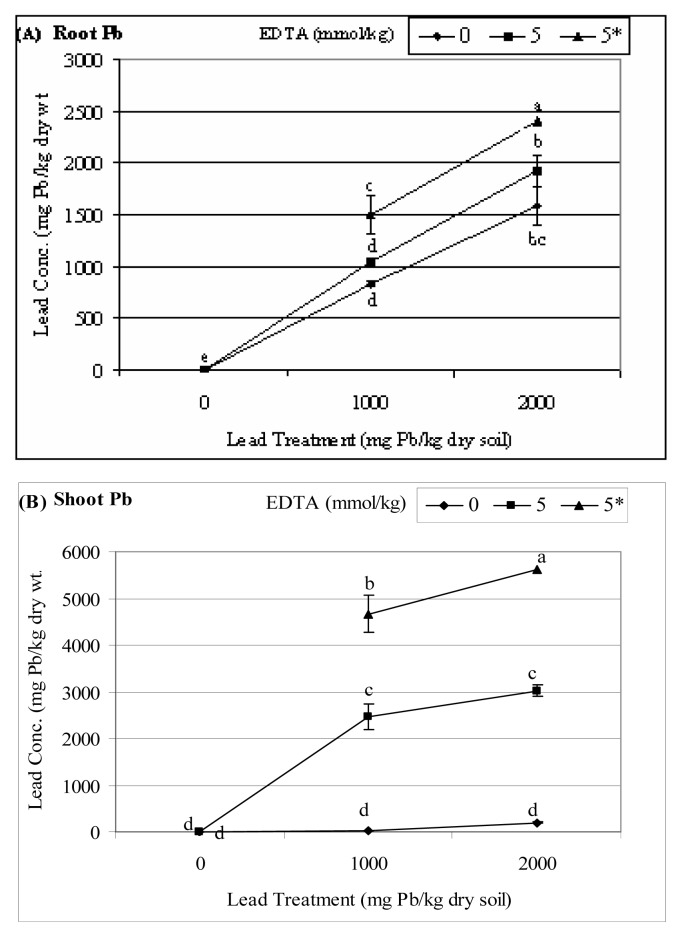
Pb accumulations in the roots increased in a dose-response manner with increasing levels of Pb treatments (Fig. 1A). Such root Pb accumulations were enhanced with EDTA amendments. Further increases in root Pb concentrations occurred due to the synergestic effects of both chelates that were applied simultaneously. When no chelates were applied, shoot Pb concentrations slightly increased with increasing levels of soil-applied Pb (Fig. 1B). Dramatic increases in shoot Pb were observed with the addition of EDTA alone. However, when both chelates were amended, increases in shoot Pb were even more remarkable.
Figure 1.
Root (A) and shoot (B) Pb concentrations of tall fescue grown at various levels of Pb and chelates. Means with a common letter do not differ significantly using Fisher’s Protected LSD test (P=0.05). An error bar indicates the standard error of the mean of 4 replications. (* indicates that an aqueous solution of acetic acid was added at the same time as the aqueous solution of EDTA).
One of the attributes of an effective phytoextraction species is its ability to maximize the amount of metal that is partitioned to the above-ground, harvestable biomass (i.e., shoots). This is indicated by the translocation index. In the absence of chelate(s), translocation indices of tall fescue plants grown at 1000 and 2000 mg Pb/kg were minimal, accounting for only 3.3% and 11.4%, respectively (Table 2). However, translocation indices dramatically increased to 66% and 73%, respectively when EDTA alone or both chelates were added simultaneously.
Table 2.
Effects of various concentrations of Pb and chelates on per cent translocation indices of tall fescue. Means with a similar letter do not differ significantly using Fisher’s Protected LSD test (p ≤0.05).
| Treatment Lead (mg Pb/kg) | EDTA (mmol/kg) | Translocation index(%) ± SEM |
|---|---|---|
| 0 | 0 | 0.0 e ± 0.0 |
| 0 | 5 Pre-harvest | 0.0 e ± 0.0 |
| 1000 | 0 | 3.3 e ± 0.4 |
| 1000 | 5 Pre-harvest | 69.8 b ± 3.0 |
| 1000 | 5* Pre-harvest | 75.6 a ± 3.0 |
| 2000 | 0 | 11.4 d ± 2.0 |
| 2000 | 5 Pre-harvest | 61.1 c ± 1.5 |
| 2000 | 5 *Pre-harvest | 70.9 ab ± 1.7 |
indicates that an aqueous solution of acetic acid (5mmol/kg dry soil) was added at the same time as the aqueous solution of EDTA; (SEM= standard error of the mean of 4 replications).
Discussion
Since total metal removal is a function of the metal concentration in the harvestable biomass (e.g., shoots), the first requisite in phytoextraction is the production of high plant biomass yield. This means that the plant species must be able to grow successfully at the contaminated site. Generally, F. arundinacea not only produced high biomass but was able to tolerate elevated levels of soil-applied Pb and chelates. There were no noticeable phytotoxic effects of Pb and/or chelates on tall fescue except for a slight reduction in root biomass (Table 1). It is known that metal phytotoxicity causes stress to the plant resulting in a reduction in biomass and eventual death (in some cases). Cunningham and Ow [34] described the presence of specific high-affinity ligands as one of the metal-resistance mechanisms existing in some plants. These ligands, which are natural metal-binding peptides known as phytochelatins and metallothioneins, make the metal less toxic to the plant, and at a certain EDTA threshold, these ligands may be activated. We are not certain whether this resistance mechanism also exists in tall fescue hence, further study is warranted. Vassil et al. [21] also demonstrated that free protonated EDTA (H-EDTA) was more phytotoxic to Brassica juncea than a Pb-EDTA complex. From earlier studies [25], we also observed the relative tolerance of wheat to Pb-EDTA complex. These previous findings support our present observation that a Pb-chelate complex was relatively nonphytotoxic to tall fescue, a monocotyledonous plant like wheat. Monocotyledonous species are usually more tolerant to metals than dicotyledonous species [35].
Root Pb accumulation increased with increasing levels of soil Pb treatments (Fig. 1A). The addition of EDTA, one week before harvest, improved Pb accumulation by the roots. However, when both EDTA and acetic acid were applied a week before harvest, there was a significant increase in root Pb accumulation especially at the highest soil Pb treatment. Majority of the absorbed Pb remained in the roots when no chelate was applied (see Fig. 1A vs. Fig. 1B). This could be due to Pb binding to ion exchangeable sites on the cell wall and extracellular deposition mainly in the form of Pb carbonates deposited on the cell wall as previously demonstrated [36]. Root depth and density are important factors in phytoextraction. It was observed that during the duration of the study, the root system of tall fescue was extensive similar to the fibrous root systems of most monocots including wheat in our previous studies [25]. Roots provide a large surface-to-volume ratio to maximize the total uptake of various elements and compounds from the soil [37]. Using hydroponics systems, Dushenkov et al. [36] concluded that root Pb absorption is a rapid process and may be the fastest component of metal removal by plants. But, the ability of plants to translocate Pb to the shoots varies much more than their ability to accumulate metals in the roots [24, 38].
One of the requisites contributing to the success of phytoextraction is the enhancement of Pb accumulation in the harvestable biomass (e.g., shoots). Vassil et al. [21] demonstrated that coordination of Pb transport by EDTA enhances the mobility within the plants of this otherwise insoluble metal ion, allowing plants to accumulate high concentrations of Pb in shoots. In this study, shoot Pb accumulation in tall fescue increased with increasing concentrations of Pb applied to the growth medium. This increase was especially remarkable in plants grown at 2000 mg Pb/kg with pre-harvest EDTA/acetic acid amendments (Fig. 1B). Lead, being a soft Lewis acid, forms a strong covalent bond not only with the soil, but with plant tissues as well [19]. It is believed that since the xylem cell walls have a high cation exchange capacity, the upward movement of metal cations are severely retarded [12]. Bringing the Pb into solution with a chelating agent, not only makes more Pb bioavailable for root uptake [18–19, 39]) but also moves the Pb that is sequestered in the xylem cell wall upwards and into the shoots.
In an earlier study, Blaylock et al. [18] demonstrated with Indian mustard (Brassica juncea) that induced phytoextraction (i.e., equivalent to pre-harvest chelate amendment in our study) brings more of the Pb ions into solution and decreases the binding of Pb by the root tissue, thereby facilitating some of the desirable characteristics of a hyperaccumulator, such as high metal uptake by the roots, and translocation of the metal from the root to the above ground shoots. We believe that EDTA enhanced Pb desorption from soil to soil solution and facilitated transport from roots to shoots as previously demonstrated in EDTA-mediated phytoextraction studies using corn, peas and Brassica juncea[18–19, 21]. Corollary studies relating the available Pb levels in soils and chelate-mediated shoot Pb uptake are currently being investigated in our laboratory. Our preliminary results indicated that with EDTA application, there is a positive correlation between bioavailable Pb levels in soil and shoot Pb accumulation (data not shown).
In a previous study by Blaylock et al. [18] using EDTA and acetic acid, the pH of the soil was decreased only slightly from 8.3 to 7.8. Similarly in our experiment, the pH of the soil before planting was 8.2, and decreased to 7.4 at harvest. Soil pH not only represents an easily determined feature of soil but is an easily managed agronomic parameter as well. Several plant nutrients become less available to plants at the extremes of pH values and other elements become available in toxic amounts [40]. Likewise, the bioavailability and plant uptake for Pb (free lead) can be accomplished by lowering soil pH. In this study, it was observed that root Pb accumulation increased as the soil pH was decreased (data not shown).
The results of this study indicated that tall fescue can be an efficient Pb-accumulating plant when coupled with other phytoextraction strategies such as lower pH, and the use of a chelate. Chelates, however, may pose environmental risks and possible contamination of the groundwater if allowed to stay long in a polluted soil, a serious concern raised in many phytoextraction studies reviewed by Lasat [39]. It is therefore likely that further technical refinements are needed on chelate-assisted phytoextraction particularly the EDTA threshold requirements for efficiency of Pb uptake. Other engineering control measures will have to be provided to prevent leaching of soluble Pb into the ground water, thereby preventing a secondary source of Pb contamination [19]. Also, limiting the resident time of the chelate in the soil by applying it a few days before harvest lessens the mobility of bioavailable metals that can potentially migrate and serve as sources of secondary pollution to the ground water [41].
Acknowledgments
This research was made possible through support provided by NASA to Jackson State University through The University of Mississippi under the terms of Grant No. NGT5-40098.
References
- 1.Lantzy R. J., Mackenzie F. T. Atmospheric trace metals: Global cycles and assessment of man’s impact. Geochim. Cosmochim. Acta. 1979;43:511–525. [Google Scholar]
- 2.Nriagu J. O. Global inventory of natural and anthropogenic emissions of trace metals to the atmosphere. Nature. 1979;279:409–411. doi: 10.1038/279409a0. [DOI] [PubMed] [Google Scholar]
- 3.Body P. E., Dolan P. R., Mulcahy D. E. Environmental lead-A review. Crit. Rev. Environ. Control. 1991;20:299–310. [Google Scholar]
- 4.Forstner U. Land contamination by metals: global scope and magnitude of problem. In: Allen H. E., Huang C. P., Bailey G. W., Bowers A. R., editors. Metal speciation and contamination of soil. CRC Press; Boca Raton, FL: 1995. pp. 1–33. [Google Scholar]
- 5.Ensley B. D. Rationale for use of phytoremediation. In: Raskin I., Ensley B.D., editors. Phytoremediation of toxic metals: Using plants to clean up the environment. John Wiley & Sons Inc; New York, NY: 2000. pp. 1–14. [Google Scholar]
- 6.Moffat A. S. Plants proving their worth in toxic metal cleanup. Science. 1995;269:302–303. doi: 10.1126/science.269.5222.302. [DOI] [PubMed] [Google Scholar]
- 7.Glass D. J. Economic potential of phytoremediation. In: Raskin I., Ensley B. D., editors. Phytoremediation of toxic metals: Using plants to clean up the environment. John Wiley & Sons Inc; New York: 2000. pp. 15–30. [Google Scholar]
- 8.McGrath S. P., Sidoli C. M. D., Baker A. J. M., Reeves R. D. The potential for the use of metal-accumulating plants for the in situ decontamination of metal-polluted soils. In: Eijsacker H.J.P., Hamers T., editors. Integrated soil sediment research: A basis for proper protection. Kluwer Academic Publ; Dordrecht, Netherlands: 1993. pp. 673–677. [Google Scholar]
- 9.Raskin I., Kumar P. B. A. N., Dushenkov V., Salt D. E. Bioconcentration of heavy metals by plants. Curr. Opin. Biotechnol. 1994;5:285–290. [Google Scholar]
- 10.Chaney R. L., Malik M., Li Y. M., Brown S. L., Brewer E. P., Angle J. S., Baker A. J. M. Phytoremediation of soil metals. Curr. Opin. Biotechnol. 1997;8:279–284. doi: 10.1016/s0958-1669(97)80004-3. [DOI] [PubMed] [Google Scholar]
- 11.Salt D. E., Blaylock M., Kumar P. B. A. N., Dushenkov V., Ensley B. D., Chet I., Raskin I. Phytoremediation: a novel strategy for the removal of metals from the environment using plants. Biotechnology. 1995;13:468–474. doi: 10.1038/nbt0595-468. [DOI] [PubMed] [Google Scholar]
- 12.Salt D. E., Smith R. D., Raskin I. Phytoremediation. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1998;49:643–668. doi: 10.1146/annurev.arplant.49.1.643. [DOI] [PubMed] [Google Scholar]
- 13.Shen Z. G., Zhao F. J., McGrath S. P. Uptake and transport of zinc in the hyperaccumulator Thlaspi caerulescens and the nonhyperaccumulator Thlaspi ochroleucum. Plant Cell Environ. 1997;20:898–906. [Google Scholar]
- 14.Ghosh S., Rhyne C. Influence of EDTA on Pb uptake in two weed species, Sesbania and Ipomoea, in hydroponic culture. J. Mississippi Acad. Sci. 1999;44:11. [Google Scholar]
- 15.Kinniburgh D. G., Jackson M. L., Syers J. K. Adsorption of alkaline-earth, transition, and heavy-metal cations by hydrous oxide gels of iron and aluminum. Soil Sci. Soc. Am. J. 1976;40:796–799. [Google Scholar]
- 16.McBride M. B. Environmental chemistry of soils. Oxford University Press; New York: 1994. [Google Scholar]
- 17.Jorgensen S. E. Removal of heavy metals from compost and soil by ecotechnological methods. Ecol. Eng. 1993;2:89–100. [Google Scholar]
- 18.Blaylock M. J., Salt D. E., Dushenkov S., Zakharova O., Gussman C., Kapulnik Y., Ensley B. D., Raskin I. Enhanced accumulation of Pb in Indian mustard by soil-applied chelating agents. Environ. Sci. Technol. 1997;31:860–865. [Google Scholar]
- 19.Huang J. W., Chen J., Berti W. R., Cunningham S. D. Phytoremediation of lead contaminated soils: Role of synthetic chelates in lead phytoextraction. Environ. Sci. Technol. 1997;31:800–805. [Google Scholar]
- 20.Ebbs S. D., Kochian L. V. Phytoextraction of zinc by oat (Avena sativa), barley (Hordeum vulgare) and Indian mustard (Brassica juncea) Environ. Sci. Technol. 1998;32:802–806. [Google Scholar]
- 21.Vassil A. D., Kapulnik Y., Raskin I., Salt D. E. The role of EDTA in lead transport and accumulation by Indian mustard. Plant Physiol. 1998;117:447–453. doi: 10.1104/pp.117.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wu J., Hsu F. C., Cunningham S. D. Chelate-assisted Pb phytoextraction: Pb availability, uptake, and translocation constraints. Environ. Sci. Technol. 1999;33:1898–1905. [Google Scholar]
- 23.Begonia G. B., Begonia M. F. T., Miller G. S., Kambhampati M. S. Phytoremediation of metal-contaminated soils: Jackson State University research initiatives. In: Centeno J. A., Collery P., Vernet G., Finkelman R. B., Gibb H., Etienne J. C., editors. Metal Ions in Biology and Medicine. Vol. 6. 2000. pp. 682–684. [Google Scholar]
- 24.Kayser A., Wenger K., Keller A., Attinger W., Felix H. R., Gupta S. K., Schulin R. Enhancement of phytoextraction of Zn, Cd, and Cu from calcareous soil: The use of NTA and sulfur amendments. Environ. Sci. Technol. 2000;34:1778–1783. [Google Scholar]
- 25.Begonia M. F. T., Begonia G. B., Butler A., Burrell M., Ighoavodha O., Crudup B. Chelate-assisted phytoextraction of lead from a contaminated soil using wheat (Triticum aestivum L.) Bull. Environ. Contam. Toxicol. 2002a;68:705–711. doi: 10.1007/s001280311. [DOI] [PubMed] [Google Scholar]
- 26.Begonia G. B., Miller G. S., Begonia M. F. T., Burks C. Chelate-enhanced phytoextraction of lead-contaminated soils using coffeeweed (Sesbania exaltata Raf.) Bull. Environ. Contam. Toxicol. 2002b;69(5):624–631. doi: 10.1007/s00128-002-0106-6. [DOI] [PubMed] [Google Scholar]
- 27.Begonia M. T., Begonia G. B., Butler A. D., Griffin U., Young C. Chemically-enhanced phytoextraction of cadmium-contaminated soils using wheat (Triticum aestivum L.) Bull. Environ. Contam. Toxicol. 2003;71(3):648–654. doi: 10.1007/s00128-003-8876-z. [DOI] [PubMed] [Google Scholar]
- 28.Begonia M. F. T., Begonia G. B., Ighoavodha M., Okuyiga-Ezem O., Crudup B. Chelate-induced phytoextraction of lead from contaminated soils using tall fescue (Festuca arundinacea) J. Mississippi Acad. Sci. 2001;46(1):15. [Google Scholar]
- 29.Tessier A., Campbell P. G. C., Bisson M. Sequential extraction procedure for the speciation of particulate trace-metals. Anal. Chem. 1979;51:844–851. [Google Scholar]
- 30.Begonia G. Comparative lead uptake and responses of some plants grown on lead-contaminated soils. J. Mississippi Acad. Sci. 1997;42(2):101–106. [Google Scholar]
- 31.Begonia G. B., Davis C. D., Begonia M. F. T., Gray C. N. Growth responses of Indian mustard [Brassica juncea (L.)Czern] and its phytoextraction of lead from a contaminated soil. Bull. Environ. Contam. Toxicol. 1998;61(1):38–43. doi: 10.1007/s001289900726. [DOI] [PubMed] [Google Scholar]
- 32.U.S. Environmental Protection Agency. Test methods for evaluating solid wastes. EPA; Washington, D.C: 1990. EPA SW-846. [Google Scholar]
- 33.Athalye V. V., Ramachandran V., D’Souza T. J. Influence of chelating agents on plant uptake of 51Cr, 210Pb and 210Po. Environ. Pollut. 1995;89:47–53. [Google Scholar]
- 34.Cunningham S. D., Ow W. D. Promises and prospects of phytoremediation. Plant Physiol. 1996;110:715–719. doi: 10.1104/pp.110.3.715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marschner H. Mineral nutrition of higher plants. Academic Press; San Diego, CA: 1995. [Google Scholar]
- 36.Dushenkov V., Kumar P. B. A. N., Motto H., Raskin I. Rhizofiltration: The use of plants to remove heavy metals from aqueous streams. Environ. Sci. Technol. 1995;29:1239–1245. doi: 10.1021/es00005a015. [DOI] [PubMed] [Google Scholar]
- 37.Kumar P. B. A. N., Dushenkov V., Motto H., Raskin I. Phytoextraction: The use of plants to remove heavy metals from soils. Environ. Sci. Technol. 1995;29:1232–1238. doi: 10.1021/es00005a014. [DOI] [PubMed] [Google Scholar]
- 38.Lombi E., Zhao F. J., Dunham S. J., McGrath S. P. Phytoremediation of heavy metal-contaminated soils: Natural hyperaccumulation versus chemically enhanced phytoextraction. J. Environ. Qual. 2001;30:1919–1926. doi: 10.2134/jeq2001.1919. [DOI] [PubMed] [Google Scholar]
- 39.Lasat M. M. Phytoextraction of toxic metals: A review of biological mechanisms. J Environ. Qual. 2002;31:109–120. [PubMed] [Google Scholar]
- 40.Bridges E. M. World soils. University Press; Cambridge: 1970. [Google Scholar]
- 41.Begonia M. T., Begonia G. B., Miller G. S., Gilliard D. Effects of chelate application time on the phytoextraction of lead-contaminated soils. Bull. Environ. Contam. Toxicol. 2004;73(6):1033–1040. doi: 10.1007/s00128-004-0529-3. [DOI] [PubMed] [Google Scholar]