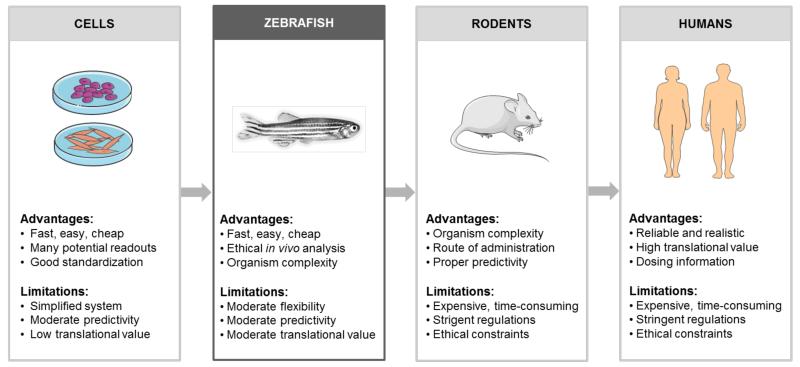
Figure 1.
Suggested workflow for translational toxicity testing of nanomedicine formulations. In general, toxicity studies commence with in vitro experiments in several different cells lines, at several different nanoparticle concentrations. Before going into patients, the material is then tested in several animal models, in particular in mice and rats. Before going into such ‘higher’ animal models, acute and chronic toxicity testing can be performed - at relatively high-throughput - using the zebrafish embryo assay. Assessing both acute and chronic effects in fish embryos which might be somewhat less informative than standard in vivo nanotoxicity screening, but it is relatively easy, more economic and arguably also more ethical. Consequently, we expect this in vivo nanotoxicity testing tool to become increasingly popular in the nanomedicine field. Figures are partially based on Servier Medical Art (www.servier.com).