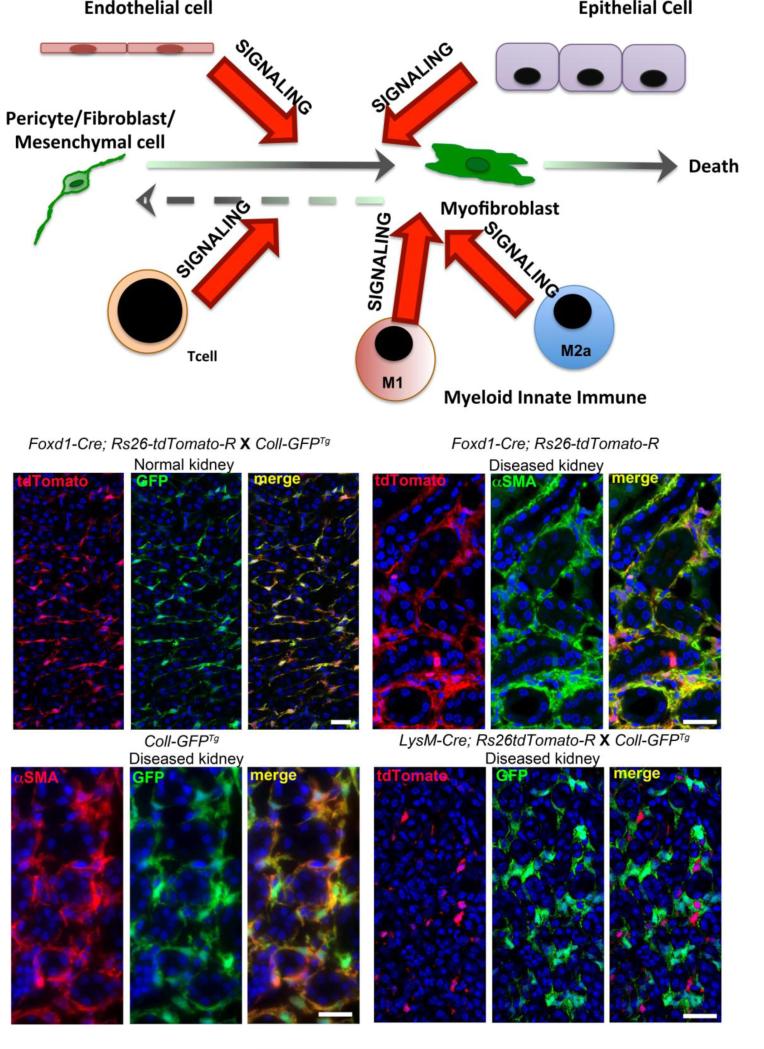
Figure. Model of Myofibroblast appearance in the kidney interstitium and images of kidneys from mice in which the fate of progenitor cells was mapped in adult kidney disease.
Schema showing the origin of kidney interstitial myofibroblasts as a population of mesenchymal cells (pericytes and resident fibroblasts) derived from FOXD1+ kidney progenitors. These cells become the major source of myofibroblasts in kidney diseases. Other cells including endothelial cells, epithelial cells, lymphocytes and macrophage populations can stimulate fibrogenesis by interacting with the resident mesenchymal cells. Left middle shows split panel image of mesenchymal cells (mainly pericytes) in normal kidney cortico-medullary junction derived from FOXD1+ progenitors (red). These cells constitutively produce Collagen I 1 protein (green). Right middle shows split panel image of the fate of those same mesenchymal cells at d7 of the Ureteral Obstruction model of kidney disease. The mapped cells (red) have expanded in number and completely overlap with αSMA+ myofibroblasts (green). Lower left shows split panel image of Collagen Iα1 protein producing (green) cells in Ureteral obstruction (d7) completely overlap with αSMA+ myofibroblasts (red). Right lower shows monocyte derived cells (due to expression of the myeloid lysosomal protein LysM) in the kidney at d7 of the Ureteral Obstruction (red). The mapped cells show no overlap with Collagen Iα1 protein producing myofibroblasts (green).