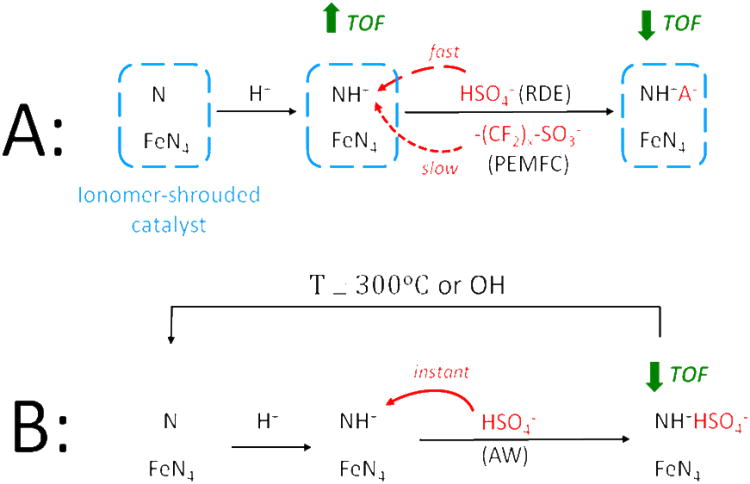
Figure 10. (scheme).
Change of the chemical state of protonable N-groups and simultaneous change of the turnover frequency (TOF) for the ORR of FeN4 catalytic sites when the Fe/N/C-catalyst is shrouded by proton-conducting ionomer (A) or directly in contact with an aqueous acidic solution (B). In case (B), the acid-washed catalyst can be reactivated either thermally or chemically. This scheme applies to Fe/N/C-catalysts obtained after a pyrolysis in NH3 with active sites labeled here as FeN4 for simplicity, but which have also been labeled as FeN2+2 previously to highlight the fact that they are hosted in micropores.44-45