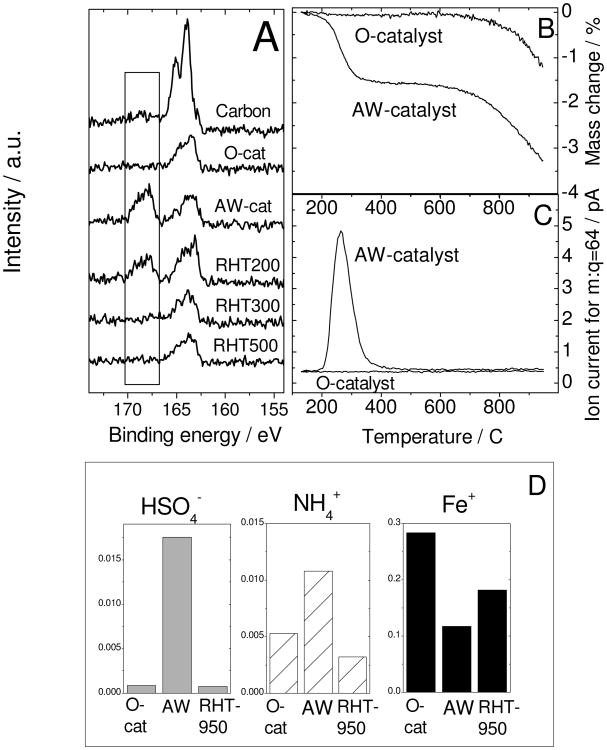
Figure 4. Identification of surface species linked to activity decay.
(A) X-ray photo-electron spectra of S2p for the pristine carbon black (label “carbon”) used to synthesize the original catalyst (O-cat), acid-washed catalyst (AW-cat) and aliquots of the latter re-heat-treated in argon for 1 h at 200, 300 or 500°C (RHT200, RHT300, RHT500, respectively).
(B) Thermogravimetry using a heating rate of 10 K min-1 and under argon flow of the O-catalyst and AW-catalyst.
(C) Ion mass-spectrometry for m:q = 64 (assigned to SO2) that was acquired simultaneously with the thermogravimetry shown in (B).
(D) Time-of-flight secondary-ion mass-spectrometry detecting HSO4−, NH4+ and Fe+ ions emitted from the top-surface layer of the O-catalyst, AW-catalyst and AW-catalyst re-heat-treated at 950°C for 1 h in argon (RHT950).