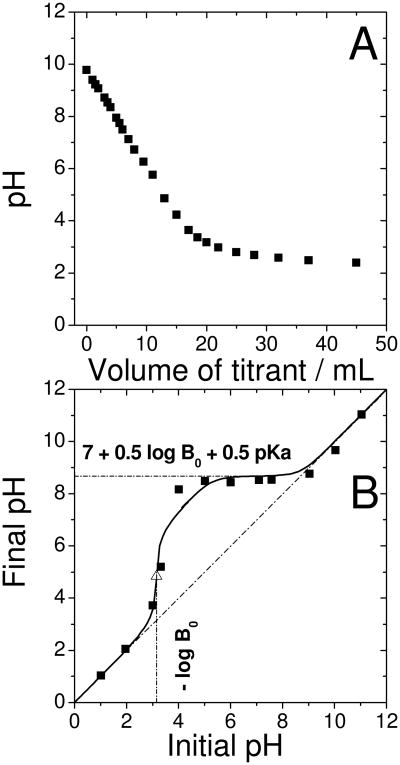
Figure 8. Investigation of basic groups on the catalyst surface by classical and Boehm titration.
(A) Classical titration: 500 mg of the original catalyst was dispersed in 25 mL of deionized water and 250 μL ethanol, then the solution was titrated by a 0.01 M aqueous solution of HClO4.
(B) Boehm titration: The original catalyst was divided into several aliquots of 20 mg and each was immersed for 20 min in 10 mL-solution of a given initial pH, then the final pH was measured (filled squares in B). The solutions were dilute aqueous solutions of either H2SO4 or NaOH.
A calculated curve corresponding to a concentration of basic groups B0 from the catalyst of 7·10-4 mol L-1 (equivalent to 7·10-4 mol L-1 × 10mL/20mg = 3.5·10-4 mol per gram catalyst) with pKa of 6.5 is also shown (solid line in (B)). In the equation, log is the base-ten logarithm function. The calculated inflection point (equivalence point) is indicated by the open triangle