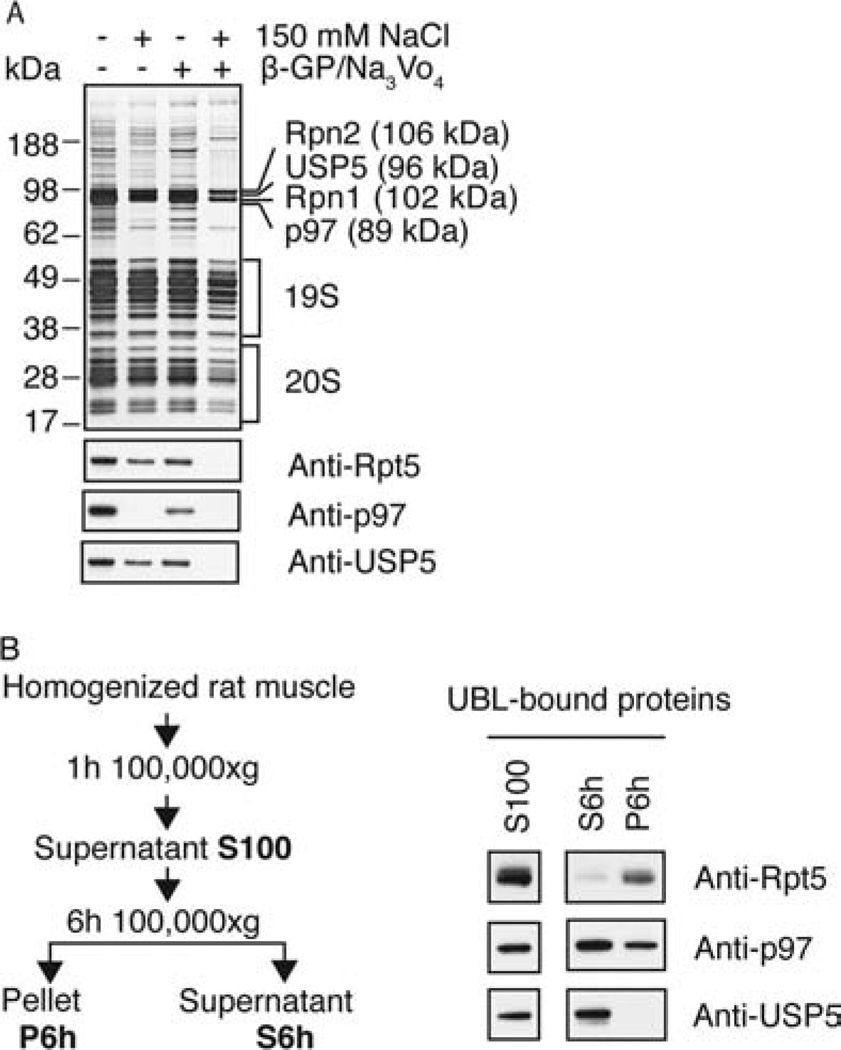
Figure 4.
In the absence of salt, USP5 and p97 complexes are purified in large amounts together with the 26S and bind to the UBL domain directly. (A) UBL-affinity preparations were carried out in the presence or absence of 150 mM NaCl and 25 mM β-glycerophosphate (β-GP)/1 mM Na3VO4. Samples containing equal amounts of proteasomal peptidase activity were analyzed by SDS–PAGE (upper panel) and Western blot (lower panels). The single bands corresponding to Rpn1, Rpn2, USP5, and p97 were identified by mass spectrometry. (B) A crude cell extract derived from 2 g of rat skeletal muscle was centrifuged for 1 h at 100000g. The pellet was discarded and the supernatant (S100) ultracentrifuged for another 6 h at 100000g. S100, S6h, and P6h were each subjected to UBL-affinity purifications and analyzed by Western blot with the indicated antibodies.