Abstract
We describe a calorimetric assay for detection of voriconazole-resistant Aspergillus fumigatus within 8 h. Among 27 genetically distinct strains, all 21 resistant and all 6 susceptible strains were correctly identified by measurement of fungal heat production in the presence of voriconazole. This proof-of-concept study demonstrates the potential of microcalorimetry for rapid detection of azole resistance in A. fumigatus.
TEXT
Voriconazole is the first-line treatment agent against invasive aspergillosis (1). The prevalence of azole-resistant Aspergillus fumigatus strains is continuously rising, highlighting the need for rapid antifungal susceptibility testing (2). Azole resistance can emerge during long-term antifungal therapy or can be induced in environmental isolates by the use of agricultural fungicides (3–5). Resistance is mainly caused by mutations in the cyp51A gene at codons G54, L98, G138, M220, G432, and G448 (6), of which L98 in combination with a tandem repeat at codon 34 is the most prevalent. The TR34/L98 mutation induces pan-azole resistance, whereas isolates harboring other mutations may remain susceptible to voriconazole and posaconazole despite resistance to itraconazole. The new environmental mutation TR46/Y121F/T289A, which at present has been described only in studies of isolates collected in the Netherlands, Belgium, and India, conveys resistance to voriconazole and variable resistance to other azoles (7–9). Different PCR assays for screening of mutations in the cyp51A gene have been described, but none of them are currently commercially available (10, 11).
The principle of microcalorimetry is based on the measuring of microbial heat production related to growth and metabolism (12). Recently, the potential of isothermal microcalorimetry was studied in a microbiology setting, including the differentiation between methicillin-susceptible and methicillin-resistant Staphylococcus aureus within 5 h (13), susceptibility testing of Aspergillus and non-Aspergillus spp. (14, 15), and evaluation of antifungal combinations against Aspergillus spp. (16). In this study, we investigated the potential of microcalorimetry for rapid detection of voriconazole resistance in A. fumigatus, using genetically characterized resistant mutants.
(Parts of the results were presented at the 23rd European Congress of Clinical Microbiology and Infectious Diseases, 27 to 30 April 2013, Berlin, Germany.)
A collection of 26 A. fumigatus clinical and environmental isolates was investigated. A. fumigatus ATCC 204305 was included for quality control (voriconazole MIC, 1 μg/ml). Sequence-based analysis of the Cyp51A gene (4, 17) showed that 20 isolates harbored the TR34/L98 mutation and two isolates a mutation at position G54 (G54E/W), exhibiting voriconazole MICs of 4 to 8 μg/ml and 0.5 μg/ml, respectively. A. fumigatus was identified based on macroscopic and microscopic morphological features and confirmed by sequencing of the internal transcribed spacer region, β-tubulin gene, and calmodulin gene (5). Voriconazole MICs were determined by broth microdilution according to EUCAST guidelines (susceptible [S] ≤ 1 μg/ml; resistant [R] > 2 μg/ml) (18).
For microcalorimetry, 3 ml Sabouraud dextrose broth (SDB) (Oxoid CM0147; Basingstoke, Hampshire, United Kingdom) containing serial dilutions of voriconazole (Pfizer Pharma AG, Zurich, Switzerland) was used, leaving 1 ml air in the headspace of the calorimetric glass ampoule. SDB without voriconazole was used for growth control. An inoculum of ∼2.5 × 105 conidia/ml was used, as determined by microscopic enumeration using a hemocytometer. Ampoules that were sealed airtight were introduced into the isothermal microcalorimeter (TAM III; TA Instruments, Newcastle, DE), and measurements were performed every 10 s at a temperature of 37°C (the accuracy of the thermostat was 10−5 °C). The detection threshold was determined at 20 μW to distinguish fungal heat production from the thermal background noise (i.e., growth media without molds). The time (in h) to reach 20 μW was monitored. Data analysis was done with the manufacturer's software (TAM Assistant; TA Instruments) and GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA). For statistical analysis, the Mann-Whitney U test and Fischer's test of equality of variances were performed.
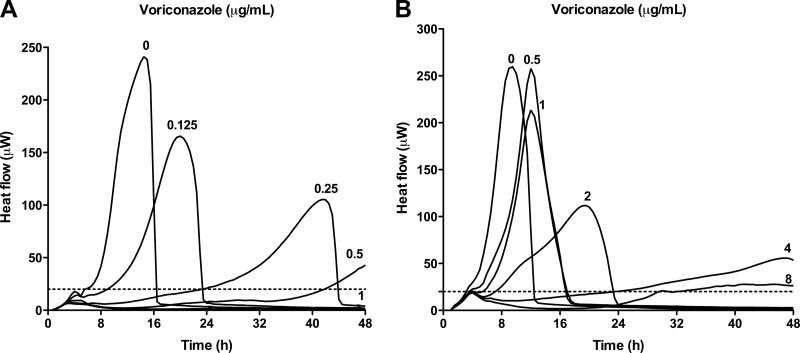
Figure 1 shows typical heat-flow curves of a voriconazole-susceptible strain (A) and a voriconazole-resistant strain (B). With increasing voriconazole concentrations, the time to reach the detection limit was delayed, with a concentration of 1 μg/ml completely inhibiting the heat flow of the susceptible strain. In contrast, growth-related heat of the resistant strain was detected even in the presence of the highest voriconazole concentration (8 μg/ml) tested.
Fig 1.
Heat-flow curves of a representative susceptible (MIC 1 μg/ml) A. fumigatus strain (A) and a resistant (MIC > 8 μg/ml) A. fumigatus strain (B) in the presence of voriconazole. The heat-detection limit of 20 μW is marked with dashed lines. Heat flow was measured for 48 h.
The mean heat-detection times (± standard deviation [SD]) in the absence of voriconazole were 5.13 ± 1.38 h and 4.23 ± 0.97 h for susceptible and resistant strains, respectively. As previously reported (19), there was no reduction in the fitness and the growth rate among the azole-resistant strains harboring TR34/L98 or G54 mutations compared with the susceptible isolates (P = 0.13). A nutrient-rich culture medium (SDB) was used in order to support optimal growth and thereby rapidly achieve a heat signal. Most recommended assays for detection of resistance are based on RPMI culture, which is a suboptimal medium for growth of clinical Aspergillus isolates, and growth conditions were therefore optimized.
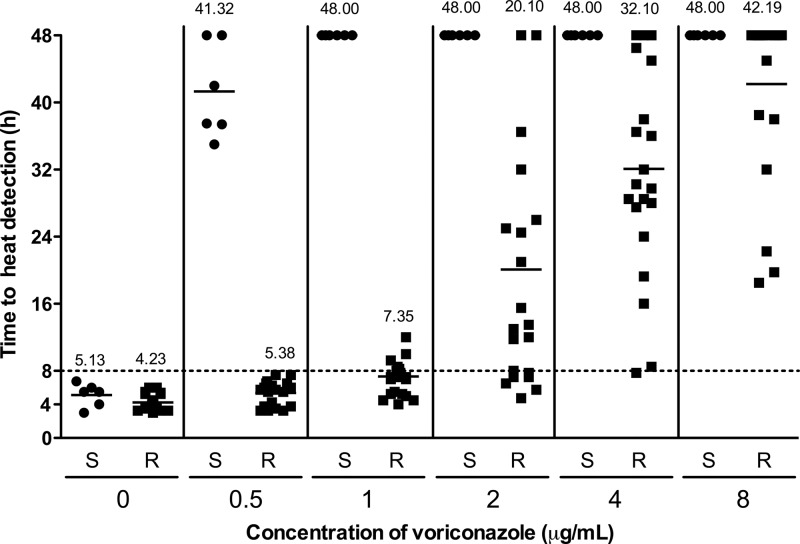
Figure 2 shows the time to heat detection of susceptible (S) and resistant (R) A. fumigatus strains in the presence of voriconazole at increasing concentrations. In the presence of 0.5 μg/ml voriconazole, the mean detection time (± SD) increased to 41.32 ± 5.65 h for susceptible strains compared to 5.38 ± 1.63 h for resistant strains (P = 0.0003). The minimal time for detection for voriconazole-resistant strains was 8 h, as indicated by the horizontal dotted line in the figure. At a concentration of 1 μg/ml, voriconazole inhibited growth of all susceptible strains for 48 h, whereas all resistant strains were detected within 12 h, with a mean detection time of 7.35 ± 2.43 h. At higher concentrations (2, 4, and 8 μg/ml) of voriconazole, the detection times for resistant strains increased to 20.10 ± 14.71 h, 32.10 ± 12.58 h, and 42.19 ± 10.19 h, respectively. For the resistant isolates, the variance in detection time was larger at voriconazole concentrations ≥ 2 μg/ml than at concentrations ≤ 1 μg/ml (P < 0.0001), indicating that there is a difference in antifungal resistance also among fungal isolates harboring the same mutation. At concentrations of 2, 4, and 8 μg/ml, the variance was not significantly different (P > 0.05) between the groups.
Fig 2.
Time to heat detection of voriconazole-susceptible (S) (n = 6, circles) and voriconazole-resistant (R) (n = 21, squares) A. fumigatus strains in the presence of serial dilutions of voriconazole from 0 to 8 μg/ml, as measured by isothermal microcalorimetry at 37°C. The numbers indicate the mean detection times in hours. The dashed horizontal line indicates the optimal time (8 h) for distinguishing azole-susceptible and azole-resistant strains in the presence of 0.5 μg/ml of voriconazole.
This proof-of-concept study demonstrates the potential of microcalorimetry for rapid detection of azole resistance in A. fumigatus. Resistant strains were distinguished from susceptible ones within 8 h in the presence of 0.5 μg/ml voriconazole in SDB. Among 27 genetically distinct fungal isolates, 21 of 21 voriconazole-resistant and 6 of 6 voriconazole-susceptible A. fumigatus isolates were correctly identified. Compared to PCR-based assays, the microcalorimetric susceptibility assay allows rapid and accurate detection of resistance without the need of knowledge of the target sequence. This is particularly important in the light of recent reports of new and previously unknown mutations leading to azole resistance (7, 8, 20–22). The importance of this report is the novel principle of susceptibility testing, which is based on heat detection instead of the currently used visible growth or molecular testing assays. By standardization and optimization of the microcalorimetric assay, azole resistance could possibly be detected directly from patient cultures rather than from pure laboratory cultures. In order to introduce microcalorimetry in a clinical laboratory setting, technical development is needed to allow automated, cost-efficient, and high-throughput susceptibility testing.
ACKNOWLEDGMENTS
J.F.M. received grants form Astellas, Merck, Basilea, and Schering-Plough. He has been a consultant to Basilea and Merck and received speaker's fees from Merck, Pfizer, Schering-Plough, Gilead, and Janssen Pharmaceutica. A.T. received grants from Pfizer and Gilead. U.F.T. declares that she has no potential conflicts of interest.
This study was supported by an educational grant from Merck Sharp and Dohme, Luzerne, Switzerland.
Footnotes
Published ahead of print 12 August 2013
REFERENCES
- 1.Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA, van Burik JA, Wingard JR, Patterson TF. 2008. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin. Infect. Dis. 46:327–360 [DOI] [PubMed] [Google Scholar]
- 2.Arikan-Akdagli S. 2012. Azole resistance in Aspergillus: global status in Europe and Asia. Ann. N. Y. Acad. Sci. 1272:9–14 [DOI] [PubMed] [Google Scholar]
- 3.Burgel PR, Baixench MT, Amsellem M, Audureau E, Chapron J, Kanaan R, Honoré I, Dupouy-Camet J, Dusser D, Klaassen CH, Meis JF, Hubert D, Paugam A. 2012. High prevalence of azole-resistant Aspergillus fumigatus in adults with cystic fibrosis exposed to itraconazole. Antimicrob. Agents Chemother. 56:869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Snelders E, Camps SM, Karawajczyk A, Schaftenaar G, Kema GH, van der Lee HA, Klaassen CH, Melchers WJ, Verweij PE. 2012. Triazole fungicides can induce cross-resistance to medical triazoles in Aspergillus fumigatus. PLoS One 7:e31801. 10.1371/journal.pone.0031801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chowdhary A, Kathuria S, Randhawa HS, Gaur SN, Klaassen CH, Meis JF. 2012. Isolation of multiple-triazole-resistant Aspergillus fumigatus strains carrying the TR/L98H mutations in the cyp51A gene in India. J. Antimicrob. Chemother. 67:362–366 [DOI] [PubMed] [Google Scholar]
- 6.Snelders E, Melchers WJ, Verweij PE. 2011. Azole resistance in Aspergillus fumigatus: a new challenge in the management of invasive aspergillosis? Future Microbiol. 6:335–347 [DOI] [PubMed] [Google Scholar]
- 7.van der Linden JW, Camps SM, Kampinga GA, Arends JP, Debets-Ossenkopp YJ, Haas PJ, Rijnders BJ, Kuijper EJ, van Tiel FH, Varga J, Karawajczyk A, Zoll J, Melchers WJ, Verweij PE. 2013. Aspergillosis due to voriconazole highly resistant Aspergillus fumigatus and recovery of genetically related resistant isolates from domiciles. Clin. Infect. Dis. 57:513–520 [DOI] [PubMed] [Google Scholar]
- 8.Vermeulen E, Maertens J, Schoemans H, Lagrou K. 2012. Azole-resistant Aspergillus fumigatus due to TR46/Y121F/T289A mutation emerging in Belgium, July 2012. Euro Surveill. 17:pii=20326 http://www.eurosurveillance.org/ViewArticle.aspx?ArticleId=20326 [PubMed] [Google Scholar]
- 9.Chowdhary A, Sharma C, Kathuria S, Hagen F, Meis JF. Azole resistant Aspergillus fumigatus with the environmental TR46/Y121F/T289A mutation in India. J. Antimicrob. Chemother., in press [DOI] [PubMed] [Google Scholar]
- 10.Spiess B, Seifarth W, Merker N, Howard SJ, Reinwald M, Dietz A, Hofmann WK, Buchheidt D. 2012. Development of novel PCR assays to detect azole resistance-mediating mutations of the Aspergillus fumigatus cyp51A gene in primary clinical samples from neutropenic patients. Antimicrob. Agents Chemother. 56:3905–3910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaassen CH, de Valk HA, Curfs-Breuker IM, Meis JF. 2010. Novel mixed-format real-time PCR assay to detect mutations conferring resistance to triazoles in Aspergillus fumigatus and prevalence of multi-triazole resistance among clinical isolates in the Netherlands. J. Antimicrob. Chemother. 65:901–905 [DOI] [PubMed] [Google Scholar]
- 12.Wadsö I. 2002. Isothermal microcalorimetry in applied biology. Thermochim. Acta 394:305–311 [Google Scholar]
- 13.Baldoni D, Hermann H, Frei R, Trampuz A, Steinhuber A. 2009. Performance of microcalorimetry for early detection of methicillin resistance in clinical isolates of Staphylococcus aureus. J. Clin. Microbiol. 47:774–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Furustrand Tafin U, Clauss M, Hauser PM, Bille J, Meis JF, Trampuz A. 2012. Isothermal microcalorimetry: a novel method for real-time determination of antifungal susceptibility of Aspergillus species. Clin. Microbiol. Infect. 18:E241–E245 [DOI] [PubMed] [Google Scholar]
- 15.Furustrand Tafin U, Meis JF, Trampuz A. 2012. Isothermal microcalorimetry for antifungal susceptibility testing of Mucorales, Fusarium spp. and Scedosporium spp. Diagn. Microbiol. Infect. Dis. 73:330–337 [DOI] [PubMed] [Google Scholar]
- 16.Furustrand Tafin U, Orasch C, Trampuz A. 2013. Activity of antifungal combinations against Aspergillus species evaluated by isothermal microcalorimetry. Diagn. Microbiol. Infect. Dis. 77:31–36. 10.1016/j.diagmicrobio.2013.06.004 [DOI] [PubMed] [Google Scholar]
- 17.Snelders E, van der Lee HA, Kuijpers J, Rijs AJ, Varga J, Samson RA, Mellado E, Donders AR, Melchers WJ, Verweij PE. 2008. Emergence of azole resistance in Aspergillus fumigatus and spread of a single resistance mechanism. PLoS Med. 5:e219. 10.1371/journal.pmed.0050219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.EUCAST 2008. EUCAST technical note on the method for the determination of broth dilution minimum inhibitory concentrations of antifungal agents for conidia-forming moulds. Clin. Microbiol. Infect. 14:982–984 [DOI] [PubMed] [Google Scholar]
- 19.Mavridou E, Bruggemann RJ, Melchers WJ, Verweij PE, Mouton JW. 2010. Impact of cyp51A mutations on the pharmacokinetic and pharmacodynamic properties of voriconazole in a murine model of disseminated aspergillosis. Antimicrob. Agents Chemother. 54:4758–4764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bader O, Weig M, Reichard U, Lugert R, Kuhns M, Christner M, Held J, Peter S, Schumacher U, Buchheidt D, Tintelnot K, Groß U. 2013. Cyp51A-based mechanisms of Aspergillus fumigatus azole drug resistance present in clinical samples from Germany. Antimicrob. Agents Chemother. 57:3513–3517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fraczek MG, Bromley M, Buied A, Moore CB, Rajendran R, Rautemaa R, Ramage G, Denning DW, Bowyer P. 2013. The cdr1B efflux transporter is associated with non-cyp51a-mediated itraconazole resistance in Aspergillus fumigatus. J. Antimicrob. Chemother. 68:1486–1496 [DOI] [PubMed] [Google Scholar]
- 22.Camps SM, Dutilh BE, Arendrup MC, Rijs AJ, Snelders E, Huynen MA, Verweij PE, Melchers WJ. 2012. Discovery of a HapE mutation that causes azole resistance in Aspergillus fumigatus through whole genome sequencing and sexual crossing. PLoS One 7:e50034. 10.1371/journal.pone.0050034 [DOI] [PMC free article] [PubMed] [Google Scholar]