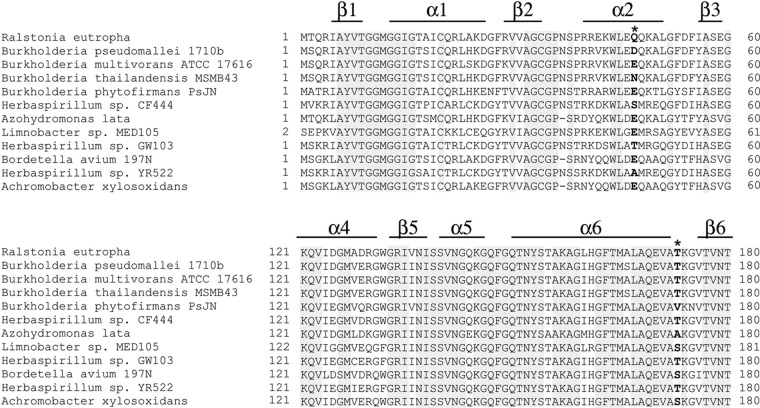
Fig 4.
Partial alignment of PhaB from R. eutropha and its homologous enzymes. Asterisks indicate the beneficial sites. The secondary structure shown is based on the crystal structure of PhaB from R. eutropha. Residue 47 locates in the nonconserved α-helix, while residue 173 locates in the random coil. Position 47 is often occupied by hydrophilic residues. Position 173 is mostly occupied by Thr, while some PhaB homologs possess a Ser residue at this position.