Abstract
This study evaluated the pharmacokinetics of topical creams containing 15% paromomycin (“paromomycin alone”) and 15% paromomycin plus 0.5% gentamicin (WR 279,396) in patients with cutaneous leishmaniasis. The investigational creams were applied topically to all lesions once daily for 20 days. Plasma samples were analyzed for simultaneous quantitation of paromomycin and gentamicin isomers and total gentamicin. Pharmacokinetic parameters for gentamicin could not be calculated because detectable levels were rarely evident. After one application, the paromomycin area under the concentration-time curve from 0 to 24 h (AUC0–24) was 2,180 ± 2,621 ng · h/ml (mean ± standard deviation [SD]) for the paromomycin-alone group and 975.6 ± 1,078 ng · h/ml for the WR 279,396 group. After 20 days of application, the paromomycin AUC0–24 and maximum concentration of drug (Cmax) were 5 to 6 times greater than those on day 1 for both treatment groups. For the paromomycin-alone group, the AUC0–24 was 8,575 ± 7,268 ng · h/ml and the Cmax was 1,000 ± 750 ng/ml, compared with 6,037 ± 3,956 ng · h/ml and 660 ± 486 ng/ml for the WR 279,396 group, respectively. Possibly due to large intersubject variability, no differences (P ≥ 0.05) in the AUC0–24 or Cmax were noted between treatment or between sites on day 1 or 20. The percentage of dose absorbed on day 20 was 12.0% ± 6.26% and 9.68% ± 6.05% for paromomycin alone and WR 279,396, respectively. Paromomycin concentrations in plasma after 20 days of application were 5 to 9% of those after intramuscular administration of 15 mg/kg of body weight/day to adults for the systemic treatment of visceral leishmaniasis. Effective topical treatment of cutaneous leishmaniasis appears to be possible with limited paromomycin and gentamicin systemic absorption, thus avoiding drug accumulation and toxicity. (The work described here has been registered at ClinicalTrials.gov under registration no. NCT01032382 and NCT01083576.)
INTRODUCTION
A combination of paromomycin and gentamicin in a topical cream formulation (WR 279,396) is being investigated for the treatment of uncomplicated cutaneous leishmaniasis (CL), a parasitic infection caused by the bite of a sandfly that results in an ulcerated skin lesion frequently 2 to 5 cm in diameter (1). Leishmaniasis has been found in more than 90 countries worldwide (2). It is estimated that the incidence of CL ranges from 0.7 million to 1.2 million cases annually (2). In the United States, CL has been found in southern Texas along the Mexican border (3) and in travelers returning from areas of endemicity (4). In the U.S. military, more than 3,100 cases of CL have been parasitologically confirmed since April 2003 at the Leishmania Diagnostics Laboratory at the Walter Reed Army Institute of Research. Although CL ultimately self-cures, the infection can create substantial morbidity due to the continued presence of a skin ulcer and the psychological impact of disfigurement (5).
Currently, there are no FDA-approved drugs for the treatment of any form of CL in the United States. Several topical agents have been investigated to treat CL, and at least one is available commercially outside the United States. The primary active drug in these topical formulations is paromomycin, an aminoglycoside antibiotic that has a structure analogous to that of the antibacterial agent neomycin. A new formulation (WR 279,396) additionally contains gentamicin, which has been shown in animal models of CL to potentiate the efficacy of the paromomycin-based topical cream formulation (6).
In clinical trials, WR 279,396 is administered once a day for 20 days for the treatment of CL. Because WR 279,396 is a combination drug, the efficacy of the combination product compared with that of the individual aminoglycosides is a mandatory part of the clinical development. Since only the paromomycin component has direct antiparasitic activity against Leishmania (7) and gentamicin is postulated to act indirectly via immunomodulation (8–10) and reduction of secondary bacterial infections in ulcerated skin lesions (11), the paromomycin-gentamicin cream (WR 279,396) is being compared to an identical formulation called “paromomycin alone,” which does not contain 0.5% gentamicin. A recent study demonstrated that both paromomycin-gentamicin (WR 279,396) and paromomycin-alone creams yielded similar cure rates for ulcerative CL, with rates of 81% and 82%, respectively, compared to rates of 58% for vehicle control (11). As part of the safety profile of this combination topical drug, the determination of the amount of the dose absorbed is important. Thus, the pharmacokinetics (PK) of both the WR 279,396 and paromomycin-alone formulations were investigated in the context of two phase 2 clinical trials, one in Peru and the other in Panama (registered at ClinicalTrials.gov under registration no. NCT01032382 and NCT01083576), and are the subject of this report. In addition, although the PK and safety of parenteral formulations of paromomycin and gentamicin separately are well known, it is not known to what extent these two drugs will be absorbed into the systemic circulation when applied topically in the investigational product formulation to an open skin wound, such as a CL lesion. The safety and efficacy of these two topical formulations are the subjects of other publications (11, 12).
MATERIALS AND METHODS
Study design.
The study was a two-site (Lima, Peru, and Panama City, Panama), randomized, double-blind, two-group trial assessing the PK, safety, and efficacy of WR 279,396 and paromomycin-alone creams in 60 subjects with uncomplicated CL. At each site, subjects were randomized in a targeted 1:1 ratio to receive either WR 279,396 (each gram of cream contains 150 mg [15% {wt/wt}] paromomycin USP base and 5 mg [0.5% {wt/wt}] gentamicin USP base) (target n = 15) or paromomycin alone (each gram of cream contains 150 mg [15% {wt/wt}] paromomycin USP base) (target n = 15) by topical application to CL lesions once daily for 20 days. Since it was desired to determine PK in different age groups, subjects were stratified by age: 5 to 17 years and ≥18 years, with no more than 18 total subjects per site randomized in any age range. The weight of the tube containing the investigational cream was measured each day after treatment, with a baseline weight taken at the beginning of treatment to estimate total exposure to the drug.
In adult subjects, on days 1 and 20, blood was collected prior to topical cream application and at 0.5 h, 1 h, 2 h, 3 h, and 4 h ± 5 min and at 8 h, 12 h, and 24 h ± 15 min after completion of cream application to determine plasma levels of paromomycin and gentamicin to calculate PK parameters. Thus, the last blood draw in this series occurred on day 21. In addition, blood was collected on days 4, 7, 12, and 17 ± 1 day before study drug application to examine trough plasma levels of paromomycin and gentamicin. A follow-up plasma sample for PK analysis was obtained from adults on day 28 ± 2 days.
Subjects under the age of 18 years had a total of four blood samples drawn (pre- and 4 h postapplication on study days 1 and 20).
Ethics.
The study was sponsored by the Office of the Surgeon General, Department of the Army, United States, and was conducted in accordance with the Belmont Principles and with all applicable laws, rules, and regulations of the United States, Peru, and Panama. The investigators adhered to the policies for protection of human subjects as prescribed in U.S. Department of Defense Instruction 3216.02, U.S. Army Regulations 70-25, and 40-7, and U.S. Food and Drug Administration regulations.
In Peru, the protocol was approved by the Independent Ethics Committee (IEC) of the Universidad Peruana Cayetano Heredia (UPCH) and the IEC of Hospital Nacional Cayetano Heredia (HNCH) in Lima City. The Ministry of Health of Peru approved importation of the study drug. In Panama, the protocol was approved by the Panamanian National Committee of Bioethics for Research, Panama City, Panama. In the United States, the protocol was approved by the Human Research Protections Office, U.S. Army Medical Research and Materiel Command, Ft. Detrick, MD. Written informed consent was obtained from all adult patients and from the legal representatives of all minors. In addition, all minors provided witnessed assent.
Measurement of plasma concentrations of paromomycin and gentamicin.
Blood (approximately 3 ml) was collected via an intravenous catheter or by venipuncture in K2 EDTA Vacutainer tubes for paromomycin and gentamicin plasma concentration determination at various time points during the study. Blood tubes were centrifuged at 3,000 rpm for 10 min at approximately 4°C, and the plasma was separated and stored at <−65°C until shipping to the Drug Studies Unit of the University of California at San Francisco for bioanalysis. Human plasma samples (0.2 ml) were analyzed for gentamicin and paromomycin with a liquid chromatography-tandem mass spectrometry (LC-MS/MS) procedure in a Micromass Quattro LC Ultima system equipped with an Allure PFP propyl column (4.6 by 50 mm, 5-μm particle size), a mobile-phase system consisting of methanol-water-trifluoroacetic acid (45:55:0.06 [vol/vol]) with 5 mM ammonium acetate and 3.0 mg ammonium phosphate dibasic per liter. Mass spectrometric detection was performed with positive ionization by (electrospray ionization (ESI) and mass scanning by multiple reaction monitoring (MRM) analysis. The LC-MS/MS method was validated for simultaneous quantitation of paromomycin and isomers of gentamicin (C1, C1a, and C2), as well as total gentamicin in plasma samples. The lower limits of quantitation (LLOQ) were 13.20 ng/ml for gentamicin C1, 12.25 ng/ml for gentamicin C1a, 21.25 ng/ml for gentamicin C2, and 50.0 ng/ml for gentamicin (total) and paromomycin. Interday assay precision ranged from a 2.77% coefficient of variation (CV) to a 4.19% CV for total gentamicin and a 2.87% CV to 3.72% CV for paromomycin.
PK analysis.
Scheduled sampling times were used in all PK parameter calculations and plots for paromomycin and gentamicin. Noncompartmental PK parameters based on the area under the concentration-time curve (AUC) and the terminal-phase exponential rate constant (λz) were estimated with a validated PK software program, WinNonLin 5.3 (Pharsight Corp., Mountain View, CA). The linear trapezoidal rule was applied to obtain AUC estimates. If plasma concentrations fell to zero, these values were also included in calculating the AUC from 0 to 24 h (AUC0–24) by the trapezoidal rule. The λz was calculated as the negative slope of the regression line for the terminal linear portion of the natural logarithm (ln)-transformed plasma concentration-versus-time curve. Selection of terminal time points was made based on the software and review of selected points. Estimates of paromomycin half-life (t1/2) that were longer than the time interval utilized to estimate the t1/2 were excluded from the PK and summary tables.
Parameters were calculated for paromomycin following topical application in adults on day 1 and day 20. No PK analysis was performed based upon gentamicin plasma concentrations, since nearly all gentamicin plasma concentrations were below quantifiable limits. As described above, λz was reported when λz could be estimated, and t1/2 was determined as ln(2)/λz. Values of AUC0–24, maximum concentration of drug (Cmax), and time to maximum concentration of drug (Tmax) for paromomycin were estimated over the 24-h period after dosing. Since the applied doses of the creams varied among subjects, values of AUC0–24 divided by the topical cream dose for that period (AUC/D) and Cmax divided by topical cream dose (Cmax/D) were also calculated. Drug concentrations in plasma for children in the table and adults in graphs were normalized for the average overall cream dose of 2.81 g.
The percentage of paromomycin absorbed systemically following topical administration (% dose) was determined from the AUC0–24 and a mean total body clearance value from the literature by Kanyok et al., 1997 (13), as follows: % dose = AUC0–24 × (paromomycin clearance of 7.32 liters/h/1.73 m2) × 100/(topical drug dose during that interval).
The total body clearance value was the mean of the two reported estimates for 12-mg/kg-of-body-weight- and 15-mg/kg single-intramuscular (i.m.)-paromomycin-dose groups (13). This calculation is correct under steady-state conditions. Thus, the % dose on day 1 was not reported. Estimates of % dose on day 20 assume steady-state conditions with similar doses during the steady-state period.
Statistical analysis.
To assess the influence of treatment (paromomycin a1lone or WR 279,396) and clinical site on the paromomycin PK, paromomycin concentrations in plasma and PK parameters were compared. Comparisons between plasma drug concentrations at each collection period between sites and treatments were performed by a one-way analysis of variance (ANOVA) (WinNonLin 5.3, Pharsight Corp., Mountain View, CA). Paromomycin PK estimates were compared with respect to treatment and site by a one-way ANOVA utilizing ln-transformed parameters.
Ordinary least-squares regression was used to examine the relationships between treatment, subject. and lesion characteristics (clinical site, age, gender, weight, body surface area [BSA], creatinine clearance, treatment prescribed, cumulative dose, and lesion area) and PK outcomes (Cmax, Tmax, AUC, Cmax/dose, and AUC/dose). Each of the characteristics was analyzed independently in a single-variable model with each PK outcome. Factors that displayed some evidence of association, defined by a P value of 0.25 or less, were included in a multivariate regression model for that PK outcome.
RESULTS
Subjects.
At the Peru site, 5 adult subjects received paromomycin alone and 4 adult subjects were treated with WR 279,396 for 20 days. For the Panama site, 8 adult subjects were treated with paromomycin-alone cream and 9 adult subjects received WR 279,396 for 20 days. For children, 18 children received paromomycin alone in Peru (n = 11) and Panama (n = 7), while 16 children were administered WR 279,396 in Peru (n = 10) and Panama (n = 6).
Demographics.
The demographics for the adult and child populations are shown in Table 1. Within the two age groups, there were no differences in age, weight, lesion size, body surface area (BSA), or creatinine clearance (CrCl) (Cockcroft and Gault [14]) between treatments or between sites.
Table 1.
Adult and child demographic characteristics and dose exposure
| Group | Treatment | No. of subjects | Value for treatment groupc |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Age, yrs | Sex, M/Fa | Wt, kg | Lesion size,b mm2 | BSA, m2 | CrCl, ml/min | Total dose, g | |||
| Adult | Paromomycin | 13 | 37 (14) | 10/3 | 74 (19) | 332 (317) | 1.83 (0.30) | 110.7 (28.5) | 53.3 (21.4) |
| WR 279,396 | 13 | 36 (12) | 8/5 | 77 (15) | 175 (163) | 1.88 (0.23) | 121.1 (44.5) | 57.8 (25.3) | |
| Child | Paromomycin | 18 | 11.4 (4.1) | 14/4 | 40.6 (17.6) | 155 (158) | 1.25 (0.37) | 101.0 (28.4) | 58.0 (20.0) |
| WR 279,396 | 16 | 10.3 (2.9) | 13/3 | 35.8 (12.7) | 112 (175) | 1.16 (0.29) | 101.7 (30.1) | 45.0 (13.9) | |
M, male; F, female.
At baseline.
Values are means (SD) except for sex of subjects.
Gentamicin PK evaluation.
No measurable gentamicin concentrations in plasma were noted in adult or child subjects receiving paromomycin-alone cream, which does not contain gentamicin. Only at a few times throughout the study with WR 279,396 did the gentamicin plasma concentrations exceed the LLOQ (13.20 ng/ml for gentamicin C1, 12.25 ng/ml for gentamicin C1a, 21.25 ng/ml for gentamicin C2, and 50.0 ng/ml for total gentamicin). These measurable gentamicin concentrations in plasma occurred 9 times on day 20 and once on day 1 (data not shown). No trend with regard to site or age group (5 adults and 5 children) was noted.
Paromomycin PK analysis.
A semilog plot of mean ± standard deviation (SD) observed paromomycin plasma concentrations ± standard deviation (SD) at the scheduled collection times is presented in Fig. 1 (day 1) and 2 (day 20). During day 1, three subjects in the paromomycin-alone group and 4 subjects in the WR 279,396 group had no measurable paromomycin plasma concentrations. Summary statistics for paromomycin PK analysis in adults on day 1 and day 20 are shown in Table 2. Estimates of λz were calculated when there were sufficient concentrations to determine a slope and were reported only if the paromomycin t1/2 was longer than the time interval utilized to estimate the t1/2 (2 profiles on day 20).
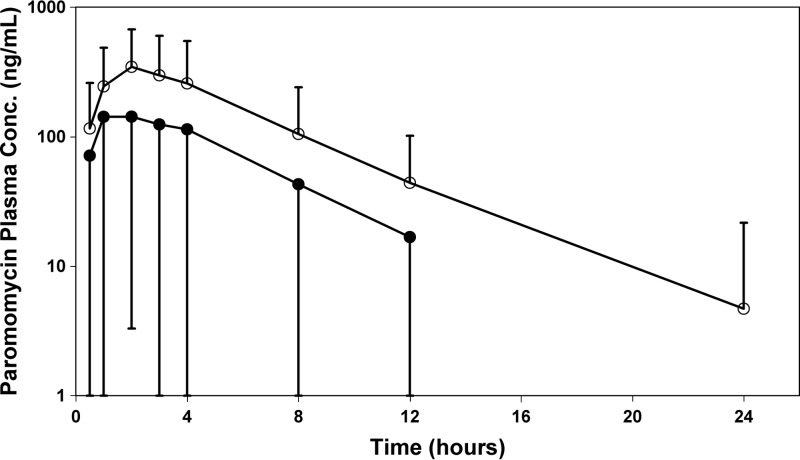
Fig 1.
Semilog plots of paromomycin plasma concentration means ± SD as a function of time following administration of paromomycin-alone (○) or WR 279,396 (●) cream on day 1 in adult subjects. Plasma concentrations are normalized to the average cream dose.
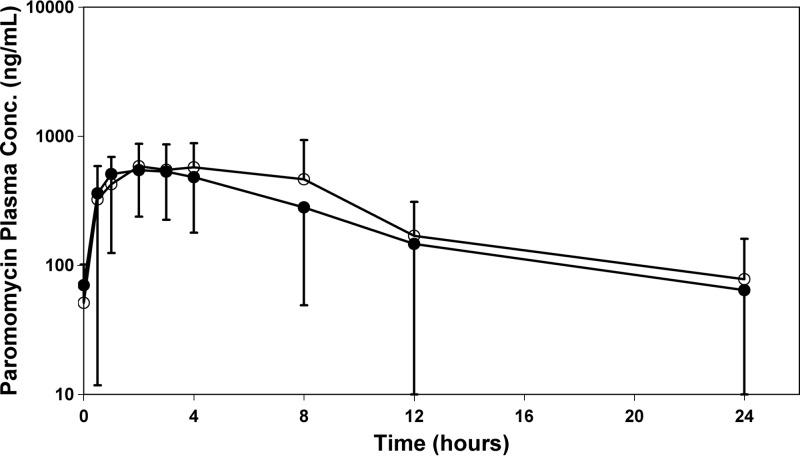
Fig 2.
Linear and semilog plots of paromomycin plasma concentration means ± SD as a function of time following administration of paromomycin-alone (○) or WR 279,396 (●) cream on day 20 in adult subjects. Plasma concentrations are normalized to the average cream dose.
Table 2.
Paromomycin PK following administration of paromomycin alone or WR 279,396 to adults on day 1 and day 20
| Day, treatment, and statistica | Dose (g) | CumDoseb (g) | Cmax (ng/ml) | Tmax (h) | AUC0–24 (ng · h/ml) | t1/2 (h) | Cmax/Dc (1/ML) | AUC/Dd (h/ML) |
|---|---|---|---|---|---|---|---|---|
| Day 1 | ||||||||
| Paromomycin | ||||||||
| No. of subjects | 13 | 13 | 13 | 10 | 13 | 9 | 13 | 13 |
| Mean value (% CV) | 2.1 (69.8) | 2.1 (69.8) | 331 (112.2) | 2.50 (38.9) | 2,180 (120.2) | 5.04 (118.4) | 135 (90.7) | 823.5 (99.0) |
| GeoMean value | 1.7 | 1.7 | 79.1 | 2.32 | 278.7 | 3.50 | 44.3 | 156.0 |
| WR 279,396 | ||||||||
| No. of subjects | 13 | 13 | 13 | 9 | 13 | 6 | 13 | 13 |
| Mean value (% CV) | 2.6 (54.7) | 2.6 (54.7) | 131 (85.7) | 2.89 (76.3) | 975.6 (110.5) | 4.15 (35.2) | 56.6 (98.5) | 344.3 (89.8) |
| GeoMean value | 2.3 | 2.3 | 35.6 | 2.33 | 125.7 | 3.96 | 18.6 | 65.69 |
| P value | ||||||||
| Day 1 ANOVA, site | 0.472 | 0.245 | 0.585 | 0.611 | 0.704 | |||
| Day 1 ANOVA, treatment | 0.478 | 0.841 | 0.597 | 0.353 | 0.500 | |||
| Day 20 | ||||||||
| Paromomycin | ||||||||
| No. of subjects | 13 | 13 | 13 | 13 | 13 | 11 | 13 | 13 |
| Mean value (% CV) | 3.4 (50.4) | 53.3 (40.1) | 1,000 (74.8) | 3.12 (73.0) | 8,575 (84.8) | 5.96 (43.5) | 281 (51.5) | 2,344 (53.3) |
| GeoMean value | 3.0 | 48.6 | 751 | 2.52 | 6,173 | 5.53 | 252 | 2,070 |
| WR 279,396 | ||||||||
| No. of subjects | 13 | 13 | 13 | 12 | 13 | 12 | 13 | 13 |
| Mean value (% CV) | 3.4 (55.4) | 57.8 (43.7) | 660 (73.7) | 2.46 (53.1) | 6,037 (65.5) | 6.94 (47.6) | 211 (61.5) | 1,926 (76.1) |
| GeoMean value | 2.8 | 52.1 | 354 | 2.07 | 2,681 | 6.21 | 135 | 1,022 |
| P value for day 20 | ||||||||
| ANOVA, site | 0.208 | 0.103 | 0.490 | 0.097 | 0.166 | 0.136 | ||
| ANOVA, treatment | 0.645 | 0.235 | 0.533 | 0.310 | 0.209 | 0.299 | ||
| P value for day 20 vs day 1, ANOVA | 0.082 | 0.003 | 0.012 | 0.005 | 0.004 | 0.007 |
GeoMean, geometric mean.
Cumulative dose.
Cmax/D, Cmax divided by administered dose of cream. ML, megaliter.
AUC/D, AUC0–24 divided by administered dose of cream.
A comparison of paromomycin concentrations in plasma by collection time showed no effect of treatment (WR 279,396 versus paromomycin alone) at any time period for both the day 1 and day 20 concentration profiles. Within each treatment group and day, no influence of study site (Peru versus Panama) was noted on observed paromomycin plasma concentrations except at two times. These times were day 20 for both adult treatment groups at 8 h and in the WR 279,396 treatment group on days 17 and 20 for adults. It must be recognized that between-site comparisons involved small numbers of subjects, with only four subjects in some Peru treatment groups.
On day 20, paromomycin AUC0-24 and Cmax estimates were greater (P ≥ 0.05) than those noted on day 1 for both treatment groups. There were no significant differences (P ≥ 0.05) in AUC0-24 or Cmax between treatment groups or sites, and this was also true for the dose-adjusted AUC/D and Cmax/D estimates.
Paromomycin plasma concentrations in children.
Table 3 presents a summary of paromomycin plasma concentrations in 34 children following topical administration of both creams. One child displayed extremely high plasma paromomycin concentrations on the first dose, with 0- and 4-h concentrations of 221 and 2,460 ng/ml. In contrast, this subject did not have detectable plasma concentrations at day 20, when it would be expected. Therefore, it is possible that these samples were mislabeled. Dropping the data for subject 313 resulted in mean paromomycin plasma concentrations of 0 and 90.5 ng/ml at these times on day 1 in children, respectively.
Table 3.
Paromomycin concentrations in plasmaa following administration of paromomycin alone or WR 279,396 to children on day 1 and day 20
| Treatment | Parameterb | Value for time point |
|||||
|---|---|---|---|---|---|---|---|
| Day 1 |
Day 1 (−sub 313c) |
Day 20 |
|||||
| 0 h | 4 h | 0 h | 4 h | 0 h | 4 h | ||
| Paromomycin | No. of subjects | 18 | 18 | 18 | 18 | ||
| Mean concn, ng/ml (% CV) | 5.00 (282.9) | 143 (98.7) | 75.7 (424.3) | 693 (424.3) | |||
| GeoMean concn | 1.73 | 94.4 | 51.8 | 505 | |||
| WR 279,396 | No. of subjects | 16 | 16 | 15 | 15 | 16 | 16 |
| Mean concn, ng/ml (% CV) | 38.9 (386.6) | 507 (326.5) | 0.0 (0.0) | 143 (82.2) | 99.0 (133.4) | 880 (98.6) | |
| GeoMean concn | 1.74 | 24.4 | 97.1 | 13.9 | 605 | ||
| P value (WR279,396 vs paromomycin) | 0.333 | 0.999 | 0.514 | 0.466 | |||
Plasma concentrations normalized to the average cream dose of 2.81 g.
GeoMean, geometric mean.
Data for subject no. 313 were omitted.
There was no significant difference (P ≥ 0.05) in paromomycin plasma concentrations between paromomycin-alone- or WR 279,396-treated children at any of the times compared on day 1 or day 20. For both treatments, plasma concentrations on day 20 appeared higher at both 0- and 4-h collection times than those on day 1. Paromomycin Cmax plasma concentrations by age group were not significantly different (P ≥ 0.05) on day 1 or 20.
Percentage of paromomycin dose systemically absorbed and accumulation.
Utilizing day 20 paromomycin PK parameters, estimates of the percentages of systemic absorption following topical administration of paromomycin alone and WR 279,396 were obtained. Since paromomycin was not given intravenously (i.v.) or intramuscularly (i.m.) to the study subjects, a paromomycin total body clearance value from a published report (13) was used in performing these estimates of the amount of paromomycin absorbed (Amt abs.) and percent of topical paromomycin dose absorbed (% dose). There are several assumptions for applying this estimation method. Since the published article by Kanyok et al. (13) presented the paromomycin PK after single i.m. dosing, it must be assumed that the extent of i.m. absorption is 100% in order to utilize the reported clearance values for the amount and % dose calculations. Also, it is assumed that on day 20, there is a steady state with respect to the paromomycin multiple-dosing rate and the resulting plasma concentration profiles.
Table 4 presents individual and summary percentages of the dose absorbed (% dose) estimates, as well as the accumulation in AUC/D for day 20 compared to day 1. Values for the % dose absorbed were highly variable, ranging from 4.15 to 24.9% for the paromomycin-alone group and 0.0 to 25.9% for the WR 279,396 group. The means ± SD for the percentage of dose absorbed were 12.0% ± 6.26% and 9.68% ± 6.05% for paromomycin alone and WR 279,396, respectively. Although not statistically evaluated, there appear to be no differences in the percentage of the topical dose systemically absorbed between the formulations. Also, there did not appear to be any trends between study sites. These % dose absorbed calculations were not performed on day 1 results, since steady state during day 1 should not be assumed. Based on the AUC/D accumulation from day 1 to day 20 as shown in Table 4, systemic steady state is not reached on day 1.
Table 4.
Predicted percentages of paromomycin systemic absorption (% dose) following administration of WR 279,396 to adult subjects on day 20
| Day, treatment, and statistic | Dose (g) | CumDosea (g) | AUC0–24 (ng · h/ml) | AUC/Db (h/ml) | Amt abs.c (mg) | % Dose | Accumd |
|---|---|---|---|---|---|---|---|
| Day 1 | |||||||
| Paromomycin | |||||||
| No. of subjects | 13 | 13 | 13 | 13 | |||
| Mean value (SD) | 2.1 (1.5) | 2.1 (1.5) | 2,180 (2,621) | 823.5 (815.1) | |||
| % CV | 69.8 | 69.8 | 120.2 | 99.0 | |||
| GeoMean value | 1.7 | 1.7 | 278.7 | 156.0 | |||
| WR 279,396 | |||||||
| No. of subjects | 13 | 13 | 13 | 13 | |||
| Mean value (SD) | 2.6 (1.4) | 2.6 (1.4) | 975.6 (1,078) | 344.3 (309.2) | |||
| % CV | 54.7 | 54.7 | 110.5 | 89.8 | |||
| GeoMean value | 2.3 | 2.3 | 125.7 | 65.69 | |||
| Day 20 | |||||||
| Paromomycin | |||||||
| No. of subjects | 13 | 13 | 13 | 13 | 13 | 13 | 10 |
| Mean value (SD) | 3.4 (1.7) | 53.3 (21.4) | 8,575 (7,268) | 2,344 (1,249) | 65.2 (48.9) | 12.0 (6.26) | 6.26 (9.34) |
| % CV | 50.4 | 40.1 | 84.8 | 53.3 | 75.1 | 52.0 | 149.2 |
| GeoMean value | 3.0 | 48.6 | 6,173 | 2,070 | 47.2 | 10.6 | 3.21 |
| WR 279,396 | |||||||
| No. of subjects | 13 | 13 | 13 | 13 | 13 | 13 | 9 |
| Mean value (SD) | 3.4 (1.9) | 57.8 (25.3) | 6,037 (3,956) | 1,926 (1,466) | 46.1 (28.7) | 9.68 (6.05) | 4.94 (4.40) |
| % CV | 55.4 | 43.7 | 65.5 | 76.1 | 62.3 | 62.5 | 89.1 |
| GeoMean value | 2.8 | 52.1 | 2,681 | 1,022 | 21.2 | 4.96 | 3.82 |
CumDose, cumulative dose over 20 days.
AUC/D, AUC0–24 divided by administered dose of cream.
Amt abs., amount of paromomycin systemically absorbed; based upon an adult paromomycin clearance of 7.32 liters/h/1.73 m2.
Accum, accumulation: (AUC/D for day 20)/(AUC/D for day 1).
The accumulation in paromomycin concentrations in plasma on day 20 compared to results on day 1 was evaluated from the ratio of 24-h AUC values corrected for dose for day 20 divided by day 1. This accumulation factor could not be calculated for all subjects, since 7 of 26 displayed plasma concentrations below the LLOQ on day 1 and 1 of 26 did so on day 20. For accumulation factors that could be determined, the mean (range) was 6.26 (0.74 to 31.9) for the paromomycin-alone group and 4.94 (1.39 to 16.0) for the WR 279,396 group. No trends were apparent between treatments or sites.
Influence of demographic factors, dose, and site on paromomycin PK parameters.
The relationships between treatment, subject, and lesion characteristics (site, age, gender, weight, BSA, creatinine clearance, treatment prescribed, cumulative dose, and lesion area) and PK outcomes (Cmax, Tmax, AUC, Cmax/dose, and AUC/dose) were examined by ordinary least-squares regression. Upon evaluation of day 1 and day 20 results both combined and separately, none of the characteristics achieved a significance level of 0.05 or less for any of the PK outcomes. When subgroups of treatment and site were examined, lesion size affected (P < 0.05) the AUC/dose and % dose absorbed values on day 20 only in the paromomycin-alone subjects. Figure 3 shows % dose absorbed versus lesion size on day 20 for both treatments, and the correlation coefficient (r) is 0.334 (P = 0.095). Examining this relationship by treatment group showed the % dose absorbed versus lesion size relationship to have r values of 0.571 (P = 0.042) for paromomycin-alone and 0.223 (P = 0.463) for WR 279,396 creams. Since there were no treatment effects, this treatment-dependent relationship may be a result of a greater lesion size range in the paromomycin-alone-treated subjects.
Fig 3.
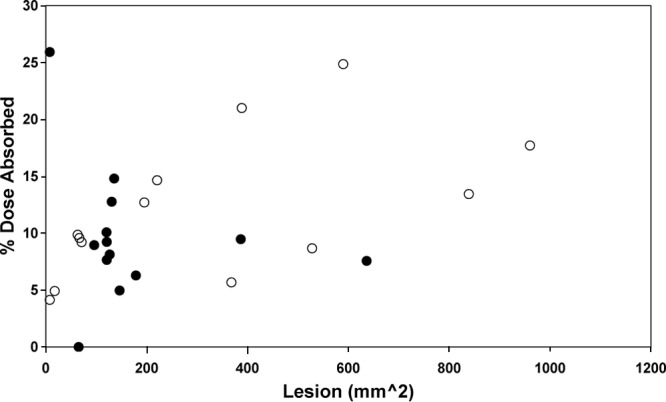
Comparisons of estimated % dose absorbed and AUC/dose versus lesion size at day 20. Paromomycin-alone cream. ○; WR 279,396 cream, ●.
DISCUSSION
The objectives of these two studies in Peru and Panama were to evaluate the PK, safety, and efficacy of WR 279,396 (paromomycin-gentamicin) and paromomycin alone in subjects with uncomplicated CL. Both creams contain 15% paromomycin. and WR 279,396 cream also contained 0.5% gentamicin. Extensive blood sampling could not be conducted in children, limiting the ability to perform a thorough PK analysis for the pediatric subjects. Blood was collected in children only prior to dosing and at the putative Tmax after the first and last days of study drug application. In addition, precise exposure to the drug was estimated based on weighing the tubes of cream before and after each day's application, such that cream that adhered to the dressing or finger of the person applying the cream would result in overestimation of the amount actually applied to the lesion and available for absorption.
Although the percentage of a topically applied drug dose that reaches the systemic circulation is generally quite low, the permeability of infected or damaged skin can be considerably greater. In the treatment of CL lesions, several weeks of topical cream application may be required, and thus there is a potential for the systemic drug accumulation of agents present in the topical cream. The PK and systemic exposure of paromomycin and gentamicin resulting from the application of these two topical creams were evaluated.
Although the PK and safety of parenteral formulations of paromomycin and gentamicin separately are well known, it is not known to what extent these two drugs will be absorbed into the systemic circulation when applied topically in this investigational product formulation to an open skin wound, such as a CL lesion. Studies of paromomycin systemic absorption after topical application in mice have shown low absorption (0.5% of dose) across intact skin but 91.5% across stripped skin (15). Also, gentamicin topical absorption is low and is reported as <2% across the intact cornea (16). Gentamicin and paromomycin have similar physiochemical properties, and, as with paromomycin, damaged skin may increase the systemic absorption of topical gentamicin (15).
Only 9 collections from more than 300 collections on day 20 had total plasma gentamicin concentration above the LLOQ. For this reason, no PK analysis was performed with the gentamicin exposure results. Among these, no pattern was noted between adults (6/9) and children (3/9). It appears that gentamicin systemic exposure from topical application of WR 279,396 cream to CL lesions is quite low and clinically insignificant.
Although quite variable, paromomycin systemic exposure following topical administration of both creams was observed, and PK analysis was performed based upon paromomycin plasma concentrations. On day 1, the Cmax varied from 0 to 1,100 ng/ml (mean ± SD = 331 ± 372 ng/ml) for paromomycin alone and 0 to 331 ng/ml (131 ± 112 ng/ml) for WR 279,396. On day 20, paromomycin Cmaxs were greater than those on day 1, with ranges of 214 to 2,240 ng/ml (1,000 ± 750 ng/ml) and 0 to 1,840 ng/ml (660 ± 486 ng/ml) for the two formulations, respectively. The 24-h paromomycin exposure (AUC0–24) on day 20 was 8,575 ± 7,268 ng/ml for paromomycin alone and 6,037 ± 3,956 ng/ml for WR 279,396.
Plasma paromomycin concentrations in children were also quite variable. On day 20, 4-h paromomycin plasma concentrations were 841 ± 794 ng/ml and 881 ± 1,170 ng/ml for paromomycin alone and WR 279,396, respectively. The trough or time zero plasma concentrations on day 20 were 85.1 ± 52.7 and 100 ± 149 ng/ml for the same. These trough and 4-h paromomycin plasma concentration are within the range noted for adults.
Using literature that has reported total-body clearances for paromomycin (13), the amount and percentage of paromomycin systemically absorbed on day 20 were estimated. For paromomycin alone, the percent dose and amount systemically absorbed were 12.0% ± 6.26% (range, 4.15 to 24.9%) and 65.2 ± 48.9 mg (11.1 to 159 mg). These same estimates for WR 279,396 were 9.68% ± 6.05% (0 to 25.9%) and 46.1 ± 28.7 mg (0 to 79.6 mg). If all adults in the study received i.m. doses of 1,000 mg/day (15 mg/kg/day × 70 kg), applying the average BSA-adjusted paromomycin clearance, the amount topically absorbed expressed as a percentage of this standard i.m. dose would be 6.52% ± 4.89% for paromomycin alone and 4.61% ± 2.87% for WR 279,396. Thus, this would be approximately 5% of a parenteral i.m. dose.
Average paromomycin concentrations in plasma over 24 h on day 20, calculated as AUC0–24/24 h, were 0.36 ± 0.30 μg/ml and 0.25 ± 0.16 μg/ml for paromomycin alone and WR 279,396, respectively. In a population PK study performed by the Institute for One World Health (IOWH), including 448 visceral leishmaniasis patients treated with 21 days of 15-mg/kg/day i.m. paromomycin, steady-state peak and trough plasma concentrations were 18.7 ± 9.45 and 1.41 ± 4.32 μg/ml in male patients and 17.6 ± 8.07 and 1.11 ± 3.89 μg/ml in female patients (16). Based upon a single i.m. dose paromomycin PK study, 15 mg/kg/day would be predicted to yield average steady-state plasma paromomycin concentrations of 4.35 ± 1.10 μg/ml (13). This comparison of plasma paromomycin concentration results in the current study to results reported or predicted in these i.m. investigations also suggests that an average of between 6 and 9% of a standard i.m. dose is being systemically absorbed after topical application of these creams to lesions.
The mean paromomycin t1/2s for both formulations and for both day 1 and day 20 ranged from 4.15 to 6.94 h. The apparent terminal t1/2 could be estimated in 9 of 26 subjects during day 1 and in 17 of 26 subjects on day 20. The fact that the t1/2 following i.m. dosing is reported as 2.64 ± 0.82 h suggested that the apparent t1/2 noted in the present topical study represents the topical systemic absorption rate, not the elimination rate (13). This apparent t1/2 did not appear to vary between formulations or between study days.
It was possible to evaluate a drug accumulation factor (measured as the ratio of dose-corrected AUCs for day 20/day 1) for 19 of the 26 subjects. This accumulation factor for AUC or exposure varied from 0.74 through 31.9, with mean values of 6.26 and 4.94 for paromomycin alone and WR 279,396, respectively. This accumulation is far greater than that expected for a drug with an apparent t1/2 of 2.64 (i.m.) or 4.15 to 6.94 (topical) h. A 7-h t1/2 and once-a-day dosing would be expected to lead to only 10% accumulation or a factor of 1.1. It is expected that with continuous topical application on the same site, skin tissue binding and uptake may become saturated, with greater portions of the later topical doses being systemically absorbed. This could explain why day 20 plasma concentrations are 5 to 6 times that observed for the first dose, even though paromomycin has a short t1/2 with little expected accumulation on multiple dosing.
Accumulation of topically applied drugs in the skin has been described previously (18, 19). For example, Testim (testosterone gel) is a clear to translucent hydroalcoholic topical gel containing 1% testosterone (Testim prescribing information; Auxilium Pharmaceuticals, Inc., 2009) that provides continuous transdermal delivery of testosterone for 24 h. Approximately 10% of the applied testosterone dose is absorbed across skin of average permeability during a 24-h period. PK studies of this drug suggested that the skin serves as a reservoir for the sustained release of testosterone into the systemic circulation. Repeated applications of topical clonidine show increased systemic availability on continued application (19).
The possible relationships between AUC and Cmaxs with the doses administered, the treatment, the clinical site, and the subject's weight, age, sex, BSA, CrCl, and lesion size were explored. Only the dose on day 1 was found to have an influence on the AUC0-24 for the same day. Subject demographic characteristics, site, and treatment were not found to affect the paromomycin PK parameters or exposure. This is consistent with a previous population study in which age, gender, height, or serum creatinine values do not alter the paromomycin PK (17).
In summary, the potential systemic absorption and exposure to paromomycin and gentamicin were investigated following topical administration of the investigational topical treatments paromomycin-alone cream and WR 279,396. In the case of gentamicin, no clinically significant systemic absorption with respect to levels associated with aminoglycoside toxicity appeared to occur in adults or children. On day 20, for WR 279,396 cream, the percent dose and amount of paromomycin systemically absorbed were estimated to be 9.68% ± 6.05% (0 to 25.9%) and 46.1 ± 28.7 mg (0 to 79.6 mg). This was similar to findings for the paromomycin-alone cream. This degree of absorption represents approximate 5 to 9% of the exposure that is expected from standard paromomycin i.m. therapy. Paromomycin exposure following topical administration on day 20 was 5 to 6 times greater than that on day 1 and was probably a result of increased systemic absorption during multiple dosing due to drug saturation of skin tissue. No demographic factors, such as age, gender, weight, CrCL, or lesion size, appeared to influence the observed systemic absorption parameters. As expected for measurements of systemic absorption following topical administration, PK parameters were highly variable and may reflect the degree of tissue damage and drug dose, which was a function of lesion size. Based upon recent clinical trials, topical treatment with this cream appears to have efficacy and safety benefits with little concern of systemic drug toxicity.
ACKNOWLEDGMENTS
This work was supported by the Department of the Army.
The investigators have adhered to the policies for protection of human subjects as described in Army Regulation 70-25.
Footnotes
Published ahead of print 22 July 2013
REFERENCES
- 1.Reithinger R, Dujardin JC, Louzir H, Pirmez C, Alexander B, Brooker S. 2007. Cutaneous leishmaniasis. Lancet Infect. Dis. 7:581–596 [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization 2010. Control of the leishmaniases. Report of a meeting of the WHO Expert Committee on the Control of Leishmaniases, Geneva, 22–26 March; WHO Technical Report Series 949. World Health Organization, Geneva, Switzerland [Google Scholar]
- 3.University of Texas Southwestern Medical Center 2008. Dermatologists identify North Texas leishmaniasis outbreak. New release Dallas: Sept. 14, 2007. University of Texas Southwestern Medical Center, Dallas, TX: http://www.utsouthwestern.edu/utsw/cda/dept353744/files/411952.html Accessed 15 August 2012 [Google Scholar]
- 4.Pehoushek JE, Quinn DM, Crum WP. 2004. Cutaneous leishmaniasis in soldiers returning from deployment to Iraq. J. Am. Acad. Dermatol. 51:S125–S128 [DOI] [PubMed] [Google Scholar]
- 5.Bern C, Maguire JH, Alvar J. 2008. Complexities of assessing the disease burden attributable to leishmaniasis. PLoS Negl. Trop. Dis. 2:e313. 10.1371/journal.pntd.0000313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grögl M, Schuster BG, Ellis WY, Berman JD. 1999. Successful topical treatment of murine cutaneous leishmaniasis with a combination of paromomycin (Aminosidine) and gentamicin. J. Parasitol. 85:354–359 [PubMed] [Google Scholar]
- 7.Neal RA, Allen S, McCoy N, Olliaro P, Croft SL. 1995. The sensitivity of Leishmania species to aminosidine. J. Antimicrob. Chemother. 35:577–584 [DOI] [PubMed] [Google Scholar]
- 8.Daneshvar H, Coombs GH, Hagan P, Phillips RS. 2003. Leishmania mexicana and Leishmania major: attenuation of wild-type parasites and vaccination with attenuated lines. J. Infect. Dis. 187:1662–1668 [DOI] [PubMed] [Google Scholar]
- 9.Daneshvar H, Burchmore R, Hagan P, Phillips RS. 2009. Leishmania major H-line attenuated under pressure of gentamicin, induces a Th1 response which protects susceptible BALB/c mice against infection with virulent L. major. Parasitology 136:1243–1250 [DOI] [PubMed] [Google Scholar]
- 10.Daneshvar H, Molaei MM, Kamiabi H, Burchmore R, Hagan P, Phillips RS. 2010. Gentamicin-attenuated Leishmania infantum: cellular immunity production and protection of dogs against experimental canine leishmaniasis. Parasite Immunol. 32:722–730 [DOI] [PubMed] [Google Scholar]
- 11.Ben Salah A, Ben Messaoud N, Guedri E, Zaatour A, Ben Alaya N, Bettaieb J, Gharbi A, Hamida NB, Boukthir A, Chlif S, Abdelhamid K, El Ahmadi Z, Louzir H, Mokni M, Morizot G, Buffet P, Smith PL, Kopydlowski KM, Kreishman-Deitrick M, Smith KS, Nielsen CJ, Ullman DR, Norwood JA, Thorne GD, MacCarthy W, Adams RC, Rice RM, Tang D, Berman J, Ransom J, Magill AJ, Grogl M. 2013. Topical paromomycin with and without gentamicin for cutaneous leishmaniasis. N. Engl. J. Med. 368:524–532 [DOI] [PubMed] [Google Scholar]
- 12.Sosa N, Capitán Z, Nieto J, Mieto M, Calzada J, Paz H, Spadafora C, Kreishman-Deitrick M, Kopydlowski K, Ullman D, McCarthy WF, Ransom JH, Berman J, Scott C, Grogl M. Randomized, double-blinded, phase 2 trial of WR 279,396 (paromomycin and gentamicin) for cutaneous leishmaniasis in Panama. Am. J. Trop. Med. Hyg., in press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kanyok TP, Killian AD, Rodvold KA, Danziger LH. 1997. Pharmacokinetics of intramuscularly administered aminosidine in healthy subjects. Antimicrob. Agents Chemother. 41:982–986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault MH. 1976. Prediction of creatinine clearance from serum creatinine. Nephron 16:31–41 [DOI] [PubMed] [Google Scholar]
- 15.Ferreira LS, Ramaldes GA, Nunan EA, Ferreira LA. 2004. In vitro skin permeation and retention of paromomycin from liposomes for topical treatment of the cutaneous leishmaniasis. Drug Dev. Ind. Pharm. 30:289–296 [DOI] [PubMed] [Google Scholar]
- 16.Trope GE, Lawrence JR, Hind VM, Everden A. 1979. Systemic absorption of topical and subconjunctival gentamicin. Br. J. Ophthalmol. 63:692–693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Institute for One World Health 2010. Application for inclusion of paromomycin in the WHO Model List of Essential Medicine. Version 12 October 2006 Institute for One World Health, San Francisco, CA: http://archives.who.int/eml/expcom/expcom15/applications/newmed/paromomycin/paromomycin.pdf [Google Scholar]
- 18.Roberts MS, Cross SE, Anissimov YG. 2004. Factors affecting the formation of a skin reservoir for topically applied solutes. Skin Pharmacol. Physiol. 17:3–16 [DOI] [PubMed] [Google Scholar]
- 19.Shaw JE. 1984. Pharmacokinetics of nitroglycerin and clonidine delivered by transdermal route. Am. Heart J. 108:217–222 [DOI] [PubMed] [Google Scholar]