Fig 1.
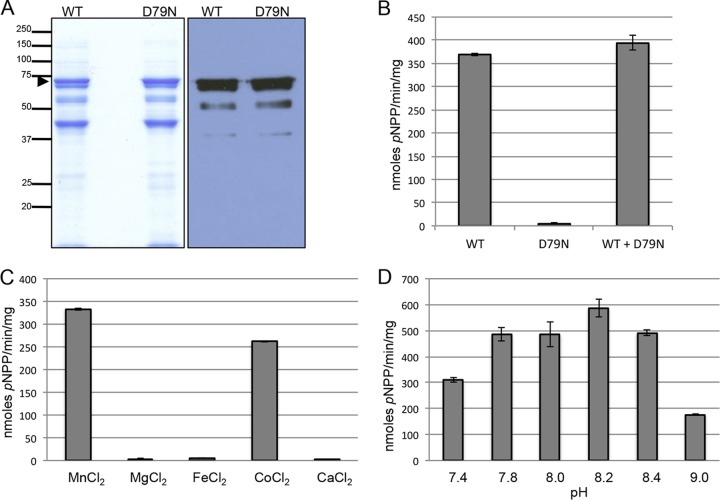
Purification and in vitro activity of MBP-PfShelph2. (A) Coomassie blue-stained SDS-PAGE gels (left; 10 μg protein per lane) and anti-MBP immunoblot (right; 1 μg of protein per lane) of affinity-purified MBP-PfShelph2. The arrowhead indicates full-length MBP-PfShelph2. Molecular masses are indicated on the left in kDa. (B) Activity of purified MBP-PfShelph2. Purified MBP-PfShelph2 WT or D79N mutant enzymes were incubated at a concentration of 10 μg/ml (100-μl total volume) with pNPP at 37°C for 30 min as described in Materials and Methods. The data are representative of three independent experiments, each performed in duplicate. The error bars indicate standard deviations. (C) Divalent metal dependency of MBP-PfShelph2. WT PfShelph2 activity was measured as for panel B with the indicated divalent metals at a concentration of 100 μM. (D) Determination of pH maximum for MBP-PfShelph2. WT MBP-PfShelph2 activity was measured as for panel B in buffer of the indicated pHs.