Fig 1.
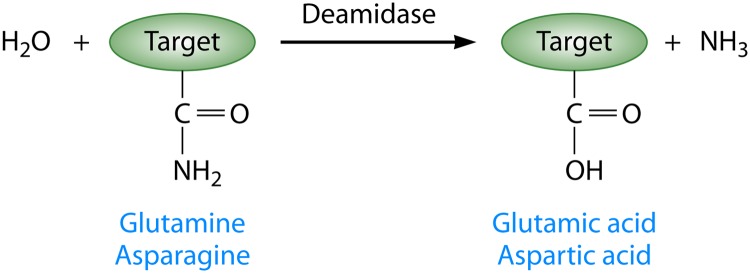
Schematic representation of enzymatic deamidation in proteins. Deamidases act on specific residues in the target protein. For all currently studied bacterial virulence factors, the targets of deamidation are glutamine side chains, which are converted to glutamic acids. However, it is also possible for the amide of the side chains of asparagines to be converted to aspartic acid. Deamidation results in an increase in the negative charge of the target protein, an increase of approximately 1 Da in the mass of the target protein, and the release of ammonia. Each of these outputs can be measured experimentally to characterize the activity of deamidases.
