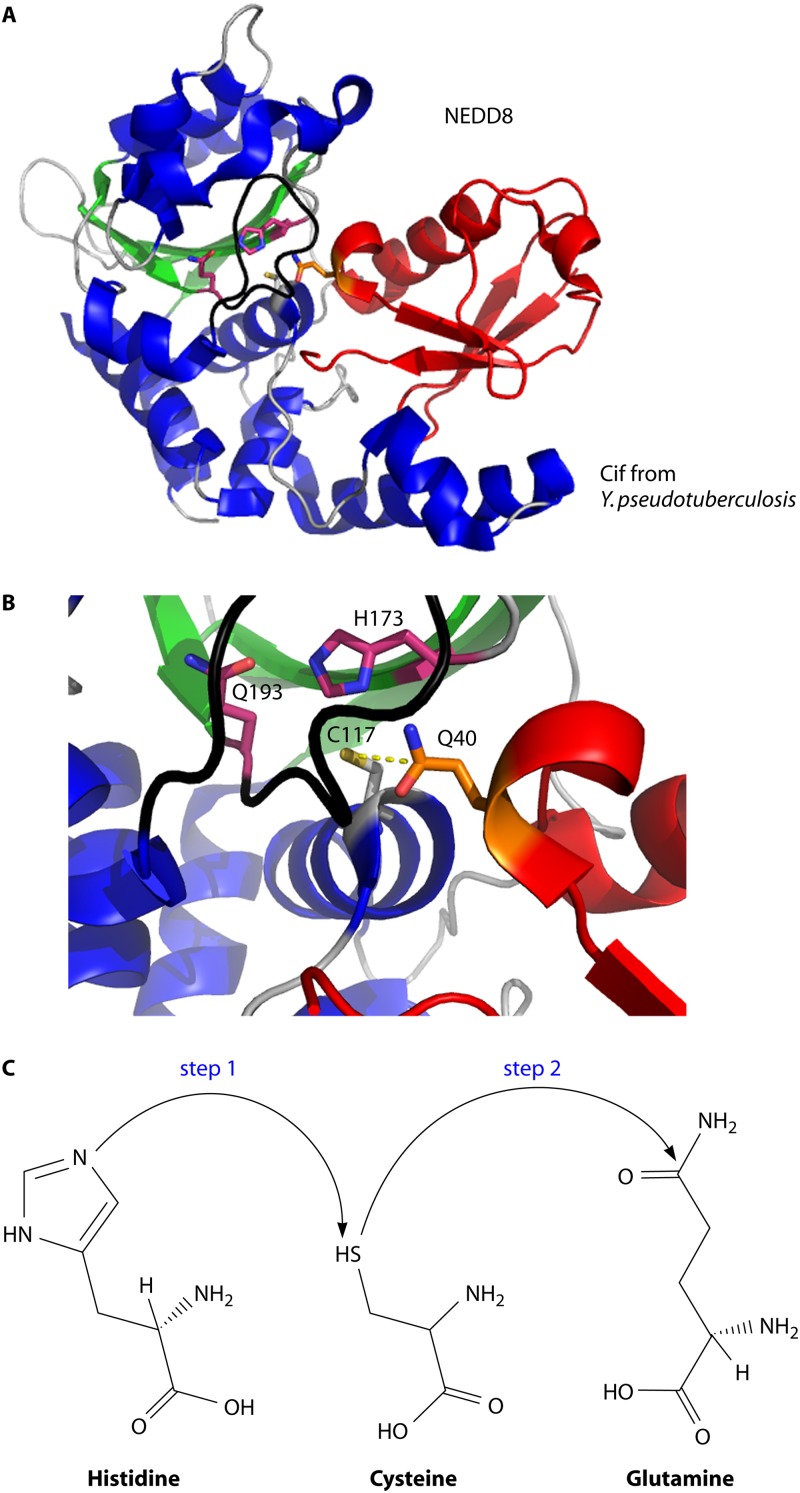
Fig 7.
Crystal structure of the CifYp deamidase-NEDD8 complex. (A) The CHYP (α-helices are shown in blue, β-sheets in green, and loops in gray)-NEDD8 (red) complex consists of an extensive interaction surface (PDB entry 4FBJ). The catalytic Cys117 position is modeled based on the CHYP(Cys117Ala)-NEDD8 complex to demonstrate the proposed molecular mechanism of deamidation. (B) Close-up of the catalytic domain of CHYP showing that the deamidation target, Gln40 of NEDD8, is positioned near the Cif catalytic residues (shown in purple). (C) The proposed molecular mechanism of deamidation involves deprotonation of the cysteine by the imidazolium group of histidine (step 1) followed by a nucleophilic attack by the thiol group of the catalytic cysteine on the δ-carbon of glutamine (step 2).