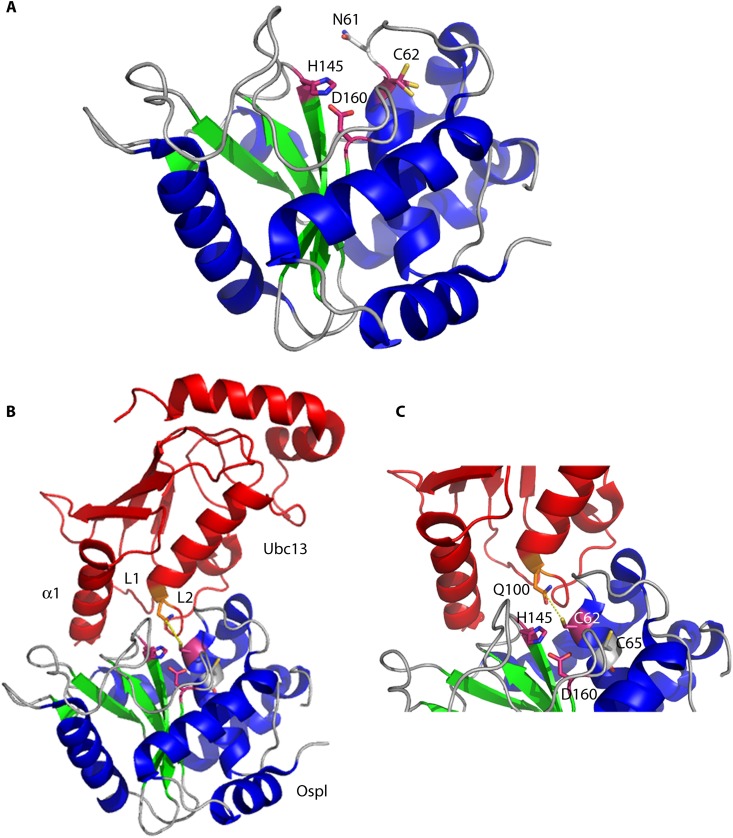
Fig 8.
Crystal structures of unbound OspI and the OspI-Ubc13 complex. (A) OspI (α-helices are shown in blue, β-sheets in green, and loops in gray) (PDB entry 3B21) forms a compact catalytic domain structurally similar to those of papain-like superfamily enzymes. The residues in the catalytic triad (Cys62, His145, and Asp160) are shown in purple. The catalytic pocket is blocked by Asn61 in free OspI. (B) The OspI(Cys62Ala)-Ubc13 (red) complex includes an extensive interaction surface involving Ubc13 α-helix 1 and loops L1 and L2 (PDB entry 4IP3). (C) Close-up of the OspI(Cys62Ala)-Ubc13 complex showing Gln100 of Ubc13 positioned adjacent to the OspI catalytic center (purple; OspI residue 62 was modeled as a cysteine to demonstrate the nucleophilic attack [yellow dashes] by the thiol group of Cys62 on Gln100 in Ubc13).