Abstract
Common presentations of tularemia include pneumonia and ulceroglandular, oropharyngeal, or typhoidal disease. Neuromeningeal involvement is extremely rare. We report a case of a severe rhombencephalitis due to Francisella tularensis. Diagnosis was possible thanks to a very precise interview, and the patient dramatically improved after specific antibiotherapy.
CASE REPORT
A 48-year-old man presented to the emergency room with a meningeal syndrome of gradual onset over the prior 9 days. His past medical history was unremarkable. On examination, he had neck stiffness, bilateral nystagmus, and a body temperature of 39°C. Brain computed tomography (CT) was normal. Cerebrospinal fluid (CSF) analysis was remarkable for a white blood cell count of 252/mm3 (92% lymphocytes), an elevated protein concentration (107 mg/dl), a normal glucose level, and a negative CSF Gram stain. Treatment with intravenous ceftriaxone, amoxicillin, and acyclovir was initiated. Acyclovir was discontinued on day 4 after two negative PCR tests for Herpesviridae. All CSF and blood cultures remained sterile. PCR tests for Listeria monocytogenes were repeatedly negative. The patient gradually worsened and was transferred to the intensive care unit (ICU) on day 10 because of multiple cranial nerve palsies, tetraparesis, confusion, and respiratory failure requiring mechanical ventilation. Cerebral magnetic resonance imaging (MRI) confirmed a rhombencephalitis (Fig. 1a) and demonstrated T2 supratentorial hypersignals and T2 hypersignals localized inside the cervical spinal cord.
Fig 1.
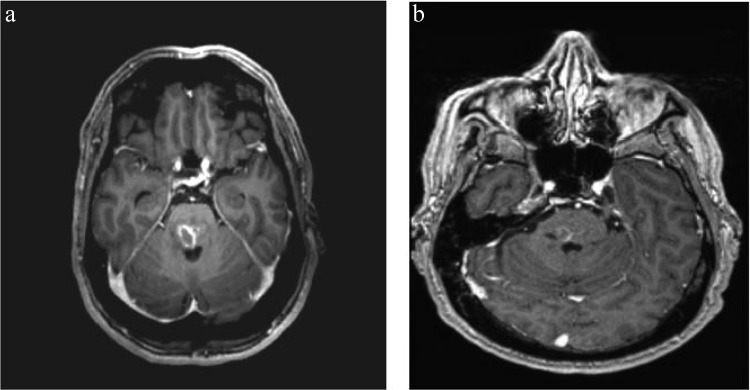
(a) Axial T1-gadolinium MR brain scan showing brainstem hypersignal; (b) axial T1-gadolinium MR brain scan control at day 68.
In-depth interview of relatives revealed regular consumption of unpasteurized milk, frequent walks in the forest, one episode of tick bite, and renovation of a henhouse during the previous weeks. Screening for most common infectious causes of encephalitis, according to French recommendations, including tests for HIV, herpes simplex virus (HSV), varicella-zoster virus (VZV), cytomegalovirus (CMV), Epstein-Barr virus (EBV), human herpesvirus 6 (HHV-6), enterovirus, Lyme borreliosis, syphilis, leptospirosis, Bartonella henselae, Mycoplasma pneumonia, and tuberculosis, were negative (1). Testing for antineutrophil cytoplasmic antibodies (ANCA) and antinuclear, antineuron, and anti-N-methyl d-aspartate receptor (NMDAr) antibodies were negative as well. Cytological examination of CSF and immunophenotyping of lymphocytes were normal. Finally, serologic testing for tularemia by enzyme-linked immunosorbent assay (ELISA) came back positive on day 26 (IgM = 200.3 IU/ml; IgG = 140.8 IU/ml), while it was negative on day 11. Neurological involvement was documented by intrathecal synthesis of Francisella tularensis IgG antibodies and confirmed by the French reference center for Francisella, using indirect immunofluorescence (IF) and microagglutinin tests (MAT) with an F. tularensis subsp. holarctica antigen. On day 11, IF IgG was 20 IU/liter, IF IgM was 0 (threshold for positivity, 80 IU/liter), and MA was 0 (threshold for positivity, 80 IU/liter). On day 26, IF IgG was 160 IU/liter, IF IgM was 160 IU/liter, and MA was 160. Cross-reaction with Yersinia and Brucella were ruled out by specific serologies. On day 27, ciprofloxacin, gentamicin, and hydroxychloroquine were initiated, the last as an enhancer of ciprofloxacin intracellular bactericidal activity in phagosomes, based on experimental data and expert opinion (2, 3). The patient gradually improved, with mild persistent facial palsy at discharge and dramatic improvement of MRI abnormalities (Fig. 1b).
Regular consumption of unpasteurized milk by a patient with rhombencephalitis of gradual onset was initially considered a strong clue for the diagnosis of listeriosis, the most common cause of subacute rhombencephalitis (4). However, all blood and CSF cultures came back negative, as did PCR tests on CSF, and the patient worsened despite prolonged, high doses of intravenous amoxicillin. The diagnosis of tularemia encephalitis, strongly suggested by the results of serologic tests, was reinforced by the dramatic improvement observed following antibacterial treatment and was adapted accordingly. The most common presentations of tularemia include pneumonia and ulceroglandular, oropharyngeal, or typhoidal disease (5), and neuromeningeal involvement is extremely rare. To our knowledge, only 22 cases of meningitis (4, 6) and 2 cases of encephalitis (1, 7) have been documented. In their recent review of 121 tularemia cases in Missouri, Weber et al. found no central nervous system involvement (8). Our patient had two main potential exposures to F. tularensis: tick bite and inhalation from the straw while renovating a henhouse. Consumption of unpasteurized milk has never been described as a potential route of Francisella infection. However, this zoonotic pathogen can infect ruminants and may be transmitted through contaminated food or beverages (9). Hence, we cannot rule out consumption of unpasteurized milk as the transmission route in this case. In conclusion, this case report of tularemic rhombencephalitis advocates for the necessity of a rigorous, step-by-step etiological search, orientated by in-depth interviews with the patient and/or the relatives.
Footnotes
Published ahead of print 31 July 2013
REFERENCES
- 1.Mailles A, Stahl JP. 2009. Infectious encephalitis in France in 2007: a national prospective study. Clin. Infect. Dis. 49:1838–1847 [DOI] [PubMed] [Google Scholar]
- 2.Rolain JM, Colson P, Raoult D. 2007. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents 30:297–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fortier AH, Leiby DA, Narayanan RB, Asafoadjei E, Crawford RM, Nacy CA, Melzer MS. 1995. Growth of Francisella tularensis LVS in macrophages: the acidic intracellular compartment provides essential iron required for growth. Infect. Immun. 63:1478–1483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hofinger DM, Cardona L, Mertz GJ, Davis LE. 2009. Tularemic meningitis in the United States. Arch. Neurol. 66:523–527 [DOI] [PubMed] [Google Scholar]
- 5.Maurin M, Pelloux I, Brion JP, Del Bano JN, Picard A. 2011. Human tularemia in France, 2006–2010. Clin. Infect. Dis. 53:e133–e141 [DOI] [PubMed] [Google Scholar]
- 6.Page J, Wittler RR. 2009. An 8-year-old boy with fever, axillary ulcerative lesion, and altered mental status. Clin. Infect. Dis. 48:1266–1267, 1327–1328 [DOI] [PubMed] [Google Scholar]
- 7.Hartman FW. 1932. Tularemic encephalitis: pathology of acute tularemia with brain involvement and coexisting tuberculosis. Am. J. Pathol. 8:57–62.3 [PMC free article] [PubMed] [Google Scholar]
- 8.Weber IB, Turabelidze G, Patrick S, Griffith KS, Kugeler KJ, Mead PS. 2012. Clinical recognition and management of tularemia in Missouri: a retrospective records review of 121 cases. Clin. Infect. Dis. 55:1283–1290 [DOI] [PubMed] [Google Scholar]
- 9.Ellis J, Oyston PC, Green M, Titball RW. 2002. Tularemia. Clin. Microbiol. Rev. 15:631–646 [DOI] [PMC free article] [PubMed] [Google Scholar]


