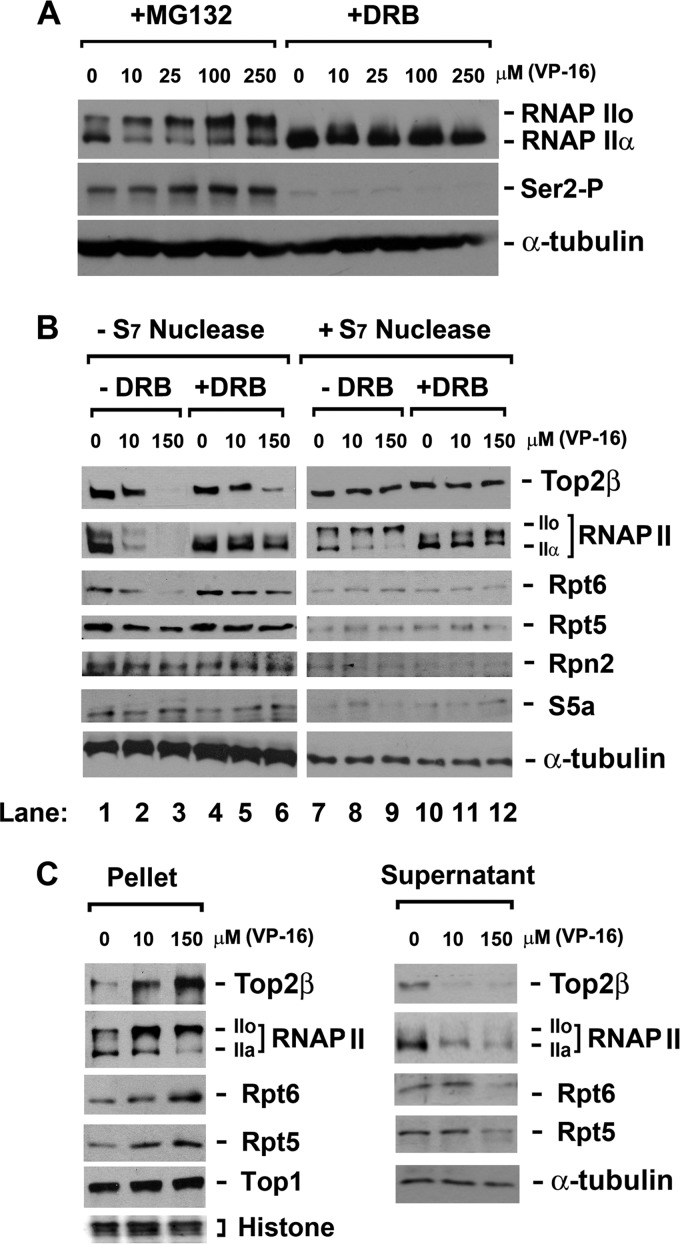
Fig 2.
VP-16 induces accumulation of RNAPIIo and 19S ATPase subunits (Rpt proteins) on chromatin. (A) VP-16 induces accumulation of hyperphosphorylated RNAP II (IIo). HeLa cells were treated with VP-16 at the indicated concentrations in the presence of either 5 μM MG132 (to prevent the degradation of RNAPII LS) or 150 μM DRB (to prevent transcription elongation) for 2 h. Cell lysates were analyzed by immunoblotting using specific antibodies against RNAPII, Ser-2-phosphorylated RNAPII, or α-tubulin. (B) VP-16 treatment increases the cross-linking of Top2β, RNAPII, and RNAPII-associated 19S ATPases to chromosomal DNA in a transcription-dependent manner. DNA-bound proteins were cross-linked to chromosomal DNA using 1% formaldehyde upon drug treatment (0, 10, or 150 μM VP-16 in the presence of MG132 or DRB for 90 min). Cell lysates were either directly analyzed by immunoblotting or subjected to S7 nuclease digestion before immunoblotting with antibodies against RNAPII, Top2β, Rpt6, Rpt5, Rpn2, S5a, or α-tubulin. (C) Chromosomal DNA-containing pellets from formaldehyde-treated cells display accumulation of Top2β, RNAPII, Rpt6, and Rpt5 in response to VP-16, while the corresponding supernatants show the opposite. HeLa cells were treated and cross-linked as described for panel B. Cell lysates were then separated into chromosomal DNA pellets and supernatants by differential centrifugation. The cell pellets were subjected to S7 nuclease digestion. Both the supernatants and the S7-digested pellets were analyzed by immunoblotting with specific antibodies against RNAPII, Top2β, Rpt6, Rpt5, Top1 (a loading control for pellets), or α-tubulin (a loading control for supernatants). Histones (another loading control for pellets) were stained by Ponceau S.