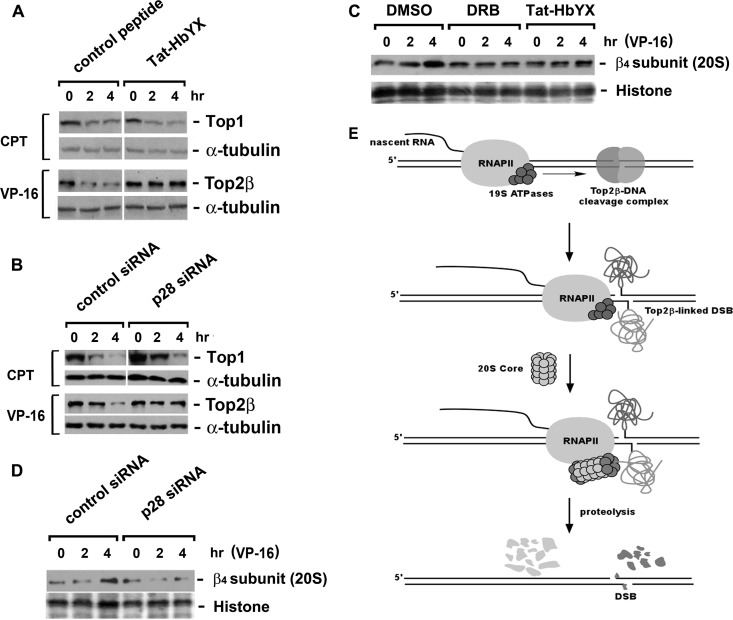
Fig 5.
VP-16-induced degradation of Top2β requires 20S-mediated assembly of 19S ATPase ring. (A) Rpt2 C terminus-derived peptide (Tat-HbYX) abolishes VP-16-induced degradation of Top2β. HeLa cells were treated with the dominant-negative peptide Tat-HbYX (100 μM) for 45 min, followed by cotreatment with 250 μM VP-16 or 25 μM CPT for 0, 2, or 4 h. Cell lysates were immunoblotted with antibody against Top1, Top2β, or α-tubulin. (B) siRNA-mediated silencing of the assembly chaperone p28 reduces VP-16-induced degradation of Top2β. HeLa cells were transfected with p28/Nas6 siRNA. Seventy-two hours posttransfection, drug treatment and immunoblotting were performed as described for panel A. (C) Both DRB and Tat-HbYX peptide prevent the recruitment of 20S proteasome to chromosomal DNA. HeLa cells were pretreated with 0.1% dimethyl sulfoxide (DMSO), DRB (150 μM), or Tat-HbYX peptide (100 μM) for 45 min, followed by cotreatment with 250 μM VP-16 for 0, 2, or 4 h. The formaldehyde cross-linking was performed, and chromosomal DNA-containing pellets were collected and processed as described in the legend to Fig. 2C, followed by immunoblotting with the anti-β4 subunit (20S proteasome) antibody. Histones were stained by Ponceau S. (D) siRNA-mediated silencing of p28 decreases chromatin-bound 20S proteasome. HeLa cells were transfected with control or p28 siRNA. Seventy-two hours posttransfection, cells were treated with 250 μM VP-16 for 0, 2, or 4 h, followed by formaldehyde cross-linking. Chromosomal DNA-containing pellets were collected, processed, and immunoblotted as described for panel C. (E) A proposed model for the interplay among elongating RNAPII, Top2β-DNA cleavage complex, and proteasome. When the elongating RNAPII stalls at the Top2β roadblock, the blocked RNAPII serves as a damage signal. The RNAPII-associated 19S ATPase recruits the 20S proteasome, resulting in the assembly of the ATPase-20S proteasome subcomplex that proteolyzes the Top2β roadblock.