Abstract
One of the most widespread clades of Mycobacterium tuberculosis worldwide, the Beijing genotype family, consists of ancient (atypical) and modern (typical) strains. Modern Beijing strains outcompete ancient strains in terms of prevalence, while reserving a higher degree of genetic conservation. We hypothesize that their selective advantage lies in eliciting a different host immune response. Bead-disrupted lysates of a collection of different M. tuberculosis strains of the modern (n = 7) or ancient (n = 7) Beijing genotype, as well as the Euro-American lineage (n = 6), were used for induction of ex vivo cytokine production in peripheral blood mononuclear cells (PBMCs) from 10 healthy individuals. Hierarchical clustering and multivariate regression analyses were used to study possible differences in production of nine cytokines. Modern and ancient M. tuberculosis Beijing genotypes induced different cytokine signatures. Overall induction of interleukin-1β (IL-1β), gamma interferon (IFN-γ), and IL-22 was 38 to 40% lower after stimulation with modern Beijing strains (corrected P values of <0.0001, 0.0288, and 0.0002, respectively). Euro-American reactivation strains induced 2-fold more TNF-α production than both types of Beijing strains. The observed differences in cytokine induction point to a reduction in proinflammatory cytokine response as a possible contributing factor to the evolutionary success of modern Beijing strains.
INTRODUCTION
DNA fingerprinting has greatly facilitated the study of the molecular epidemiology of tuberculosis, while disclosing the phylogeny of the Mycobacterium tuberculosis complex. The first genotype family described was the Beijing clade (1), later recognized as the most important part of the East Asian lineage (2). Strains of the widespread Beijing family are of particular interest due to their established association with drug resistance, increased virulence in animal models, and association with infection of younger patients (3), the last of which points to an increased relative reproductive fitness (4).
The vast majority of circulating Beijing strains is thought to belong to a conserved type genetically, as first described based on IS6110 restriction fragment length polymorphism (IS6110 RFLP) analysis (5) and recently confirmed by whole-genome sequencing (6). These so-called “modern” Beijing strains represent 65 to 95% of Beijing strains in most areas, including China (7), Russia (8), Taiwan (9), South Africa (10), Europe (7), and the United States (11). Modern Beijing strains were first named “typical” by the presence of a typical pattern of one or two copies of IS6110 in the NTF chromosomal region. In contrast, this insertion was found to be absent in the “atypical” or ancient Beijing strains. These strains seem in fact to represent a genetically diverse group (6). It is only in Japan and South Korea that the ancient strains are still highly prevalent, although they form a declining majority (12, 13).
The high prevalence and degree of genetic conservation of modern Beijing strains suggest that they possess a selective advantage over ancient Beijing strains and other M. tuberculosis genotypes. Drug resistance most likely occurred on several independent occasions (14) and cannot be linked definitively to one of the strain subtypes (15, 16). One possible explanation for their success is that modern Beijing strains induce a different, less effective host immune response than that with ancient Beijing strains. Although several studies have reported differences in immune responses after infection by Beijing genotype strains, none has performed an extensive comparison of immune responses induced by modern and ancient Beijing strains. The aim of our study, therefore, was to examine if the epidemiological success of modern M. tuberculosis Beijing strains is paralleled by a distinctive cytokine production profile. Our strain selection comprised a widespread geographic area and also included Euro-American strains isolated from persons with endogenous reactivation cases in the Netherlands. We identified differences in innate immune responses between modern and ancient M. tuberculosis Beijing strains that may help to explain their evolutionary success.
MATERIALS AND METHODS
Mycobacterium tuberculosis strains.
Twenty M. tuberculosis strains were selected from the reference database of clinical isolates of the Dutch Health and Environment Institute (RIVM) in Bilthoven, the Netherlands. Fourteen had been spoligotyped previously as Beijing strains. Using IS6110 PCR analysis of the NTF region, these strains were further subdivided into modern (n = 7) and ancient (n = 7) Beijing strains (17). A third group consisted of six strains isolated from elderly (>70 years of age) Dutch tuberculosis (TB) patients. On the basis of IS6110 RFLP typing, which is applied routinely to all TB cases in the Netherlands, all six had unique profiles and were assumed to represent endogenous reactivation from remote infections decades ago. Spoligotyping designated these strains to the Haarlem and T spoligotypes of the Euro-American lineage (Fig. 1). All strains were grown in Middlebrook 7H9 medium in one batch for 3 weeks. Ancient Beijing strains grew to a median optical density (OD) of 0.47 (range, 0.32 to 0.61), and modern Beijing strains grew to a median OD of 0.43 (range, 0.32 to 0.57), but the Euro-American reactivation strains grew less efficiently and reached a median OD of 0.27 (range, 0.15 to 0.55). The strains were washed two times in phosphate-buffered saline (PBS), heat killed, and then disrupted using a bead beater, after which the concentration was measured using a bicinchoninic acid (BCA) protein assay to standardize the concentration used in the immunological experiments.
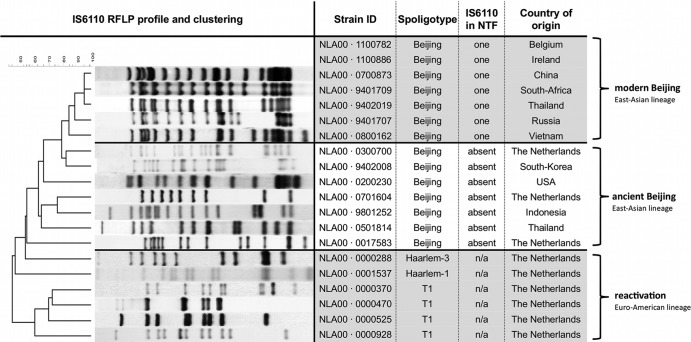
Fig 1.
Mycobacterium tuberculosis strains used for this study, with genetic markers and countries of origin. IS6110 RFLP profiles and clustering are shown, as well as typing by spoligotyping and determination of the presence of IS6110 in the NTF region. Note that all reactivation strains were isolated from Dutch patients over 70 years of age. n/a, not available.
Stimulation experiment with human PBMCs.
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats purchased from the Sanquin Bloodbank Nijmegen. Healthy volunteers gave their written informed consent for the use of their blood for scientific purposes, as approved by the Ethics Committee of Radboud University Medical Centre, Nijmegen, The Netherlands. Donations occurred anonymously, and therefore no tuberculosis skin test or gamma interferon (IFN-γ) release assay could be performed, but the present incidence of TB in the indigenous Dutch population is extremely low (4/100,000), and M. bovis BCG vaccination is not part of the routine vaccination program. Isolation was performed using Ficoll-Paque, involving separation by a density gradient followed by three wash steps in PBS and resuspension in RPMI 1640 supplemented with Glutamax, pyruvate, and gentamycin. Subsequently, 100 μl of PBMCs (5 × 106/ml) and 50 μl of stimulus at 4 times the designated final concentration were added in duplicate to a 96-well round-bottom plate, together with 50 μl of RPMI, or human pooled serum in case of a 7-day stimulation. Heat-killed Candida hyphae were used as a positive control. The plates were incubated for 24 h, 48 h, or 7 days at 37°C in a 5% CO2 environment, after which they were spun at 700 × g for 8 min. Supernatants were collected and stored at −20°C. Preliminary studies were performed using two modern and two ancient strains grown in different batches to define the dose-response relationship and select the most appropriate stimulatory concentration of bead-disrupted M. tuberculosis, based on the signatures of all cytokines tested. Afterwards, five experiments, involving two healthy volunteers each, were performed with a selection of 20 strains. Enzyme-linked immunosorbent assays (ELISAs) were performed batchwise following the manufacturer's protocols for measuring cytokines in supernatants. The following lengths of stimulation for the different cytokines were based on previous experiments: for interleukin-1β (IL-1β), IL-1 receptor antagonist (IL-1Ra), transforming growth factor beta (TGF-β), tumor necrosis factor alpha (TNF-α) (all from R&D Systems, Minneapolis, MN), and IL-6 (Sanquin, Amsterdam, the Netherlands), 24 h; for IFN-γ and IL-10 (Sanquin), 48 h; and for IL-17 and IL-22 (R&D Systems), 7 days.
Data analysis and statistics.
Hierarchical clustering analysis was performed to investigate whether modern and ancient Beijing strains induced different cytokine signatures. Data points for 20 strains times 10 donors per cytokine were ln transformed and then normalized by subtracting each value from the mean response per cytokine per donor and dividing this value by the standard deviation. This resulted in a table with standardized donor responses per cytokine in rows and different strains in columns. Using the Pearson correlation, a distance measure was calculated, on which clustering was performed with the weighted-pair group method using average linkages (WPGMA) as the linkage method, using the freeware program J Express 2012 (18).
Multivariate regression on the ln-transformed cytokine data was used to determine the contributions of individual cytokines to the difference between the strain groups. In the multivariate regression, groups were compared based on their genetic classification, unbiased for results of the clustering hierarchy, to test the hypothesis that modern Beijing strains, on average, induced a different immune response. Fixed factors were strain type (modern Beijing, ancient Beijing, or Euro-American reactivation) and donor, with the latter used to correct for donor variability. For the nine cytokines, predicted means for strain types and differences between those means with respective confidence intervals were calculated with Stata MP, version 12.1. Differences in confidence intervals were plotted on a radar graph with a logarithmic axis. Reported P values and confidence intervals were adjusted by the Bonferroni correction to correct for multiple comparisons. For clarification, relative percentages of differences were added to the radar graph and, for the reported table values, were transformed as an exponent of e to obtain the prediction of the mean in pg/ml.
RESULTS
Strains.
All strains were fully susceptible to all first-line antituberculosis drugs. See Fig. 1 for other characteristics. Using a standardized stimulation model with bead-disrupted M. tuberculosis and isolated PBMCs, we first explored concentrations of 0.1, 1.0, and 10 μg/ml M. tuberculosis, looking for a single concentration associated with reasonable induction of all cytokines evaluated. We chose a final concentration of 5 μg/ml, as this was expected to most clearly show differences between groups for all cytokines of interest. Production of TNF-α, IL-1β, IL-6, IL-10, IL-17, and IFN-γ by PBMCs from 4 donors was almost identical when different concentrations of an H37Rv control strain that was grown in two different laboratories (the Dutch National TB Reference Centre and Leiden University Medical Centre) (see Fig. S1 in the supplemental material) were used.
Cytokine signature.
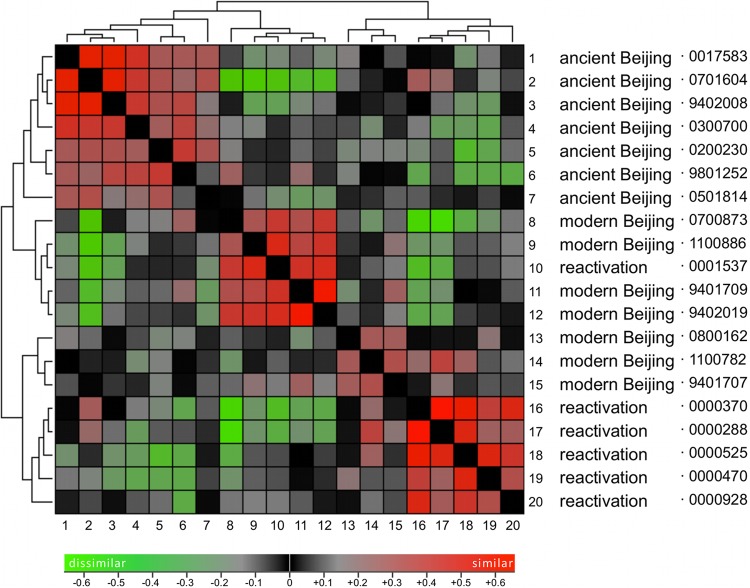
The hierarchical clustering of cytokine signatures revealed different clusters of strains that grossly followed the distinction of modern versus ancient Beijing strains and the Euro-American reactivation strains. This study aimed explicitly to explore differences between groups of strains that have shown important epidemiological differences in terms of recent spread and genetic heterogeneity. Indeed, the ancient Beijing strains clustered separately from the epidemiologically more successful and genetically more conserved modern Beijing strains. In the hierarchical clustering, this group of modern Beijing strains was divided into two groups, one of which also harbored one Euro-American reactivation strain, while the other Euro-American strains clustered as a fourth cluster (Fig. 2). Multivariate regression was applied to the groups of strains as defined based on their genetics, and statistically significant differences in cytokine responses were found between modern Beijing strains and the other two groups for IL-1β, IL-1Ra, IFN-γ, and IL-22 (Fig. 3A and B).
Fig 2.
Hierarchical clustering of modern and ancient Beijing strains and Euro-American reactivation strains. The figure shows clustering of the virtual distances between the different M. tuberculosis strains based on the cytokines they induce in PBMCs. The distance matrix shows similarity (red) and dissimilarity (green) between cytokine signatures of the respective strains. Analysis was performed on ln-transformed and normalized data with the open source software J Express (17). See the text for further details.
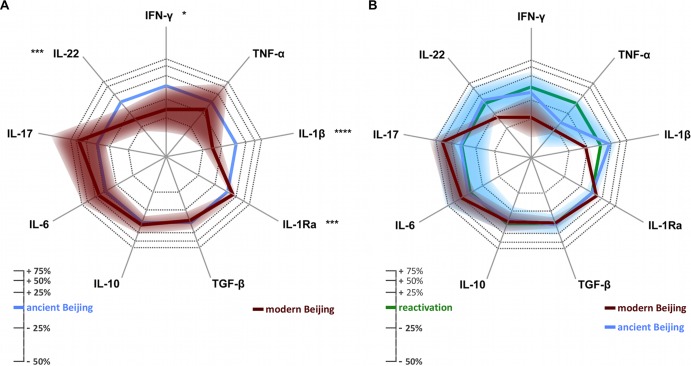
Fig 3.
(A) Differences in cytokine response after stimulation with modern (red) compared to ancient (blue) Beijing strains. (B) Differences in cytokine response after stimulation with modern (red) and ancient (blue) Beijing strains compared to Euro-American reactivation strains (green). Relative cytokine responses were calculated by multivariate regression. The shaded areas show the confidence intervals, which were adjusted by the Bonferroni correction to take into account the multiple comparisons made. The axis is on the ln scale; corresponding percentages are shown in the legend. A lower response is indicated by projection toward the center of the figure. Asterisks indicate the significance of the differences, as follows: *, P < 0.05; ***, P < 0.001; ****, P < 0.0001.
Individual cytokines.
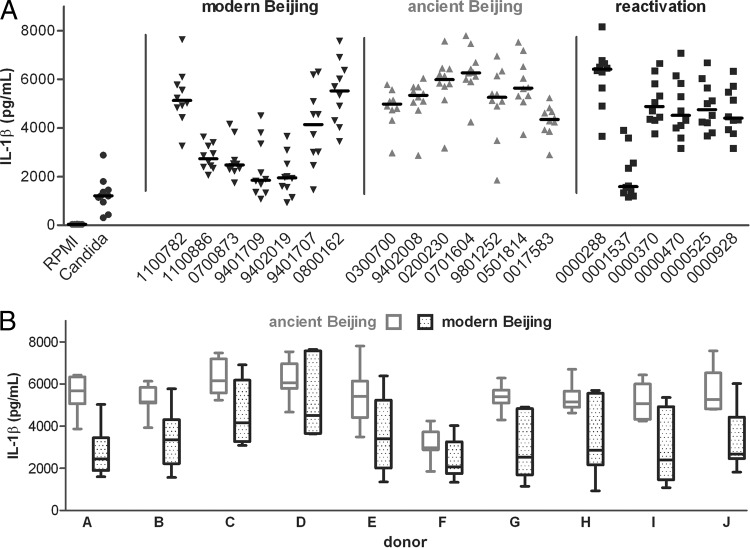
The most striking difference in cytokine production was observed for induction of IL-1β, which is essential in the protective host defense against M. tuberculosis (Fig. 3A and Table 1). Overall, modern Beijing strains induced significantly lower IL-1β concentrations after 24 h than those of ancient Beijing strains (estimated means, 3,115 versus 5,184 pg/ml) (P < 0.0001) and reactivation strains (4,250 pg/ml) (Fig. 3A and B). Moreover, compared to ancient Beijing strains, modern Beijing strains induced significantly more IL-1Ra (5,094 versus 4,501 pg/ml) (P = 0.0001). Because IL-1Ra antagonizes the effect of IL-1β, this will further deplete the biologically active fraction of IL-1β after stimulation with modern Beijing strains. Production of all cytokines, including IL-1β and IL-1Ra, showed considerable between-strain and between-donor variability (Fig. 4A). However, at the level of the individual donors, the amount of IL-1β was consistently smaller, and that of IL-1Ra was consistently larger, for modern than for ancient Beijing strains in cells from all 10 donors (Fig. 4B; see Fig. S4B in the supplemental material).
Table 1.
Cytokine induction in modern and ancient Beijing strains and Euro-American reactivation strainsa
| Cytokine | Length of stimulation | Predicted mean (95% CI) (pg/ml) |
% Difference for modern Beijing vs ancient Beijing strains | P value | % Difference for modern Beijing vs Euro-American reactivation strains | P value | % Difference for ancient Beijing vs Euro-American reactivation strains | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Modern Beijing strains | Ancient Beijing strains | Euro-American reactivation strains | ||||||||
| IFN-γ | 48 h | 48 (40–57) | 80 (67–95) | 90 (76–107) | −40 | 0.0288 | −47 | <0.0001 | −12 | 1 |
| TNF-α | 24 h | 300 (252–357) | 365 (272–490) | 623 (535–725) | −18 | 1 | −52 | <0.0001 | −41 | 0.3136 |
| IL-1β | 24 h | 3,115 (2,954–3,285) | 5,184 (4,823–5,572) | 4,250 (4,082–4,424) | −40 | <0.0001 | −27 | <0.0001 | +22 | 0.0036 |
| IL-1Ra | 24 h | 5,094 (4,971–5,220) | 4,501 (4,329–4,681) | 4,531 (4,333–4,738) | +13 | 0.0001 | +12 | 0.0010 | −1 | 1 |
| TGF-β | 24 h | 2,175 (2,067–2,289) | 2,264 (2,080–2,465) | 2,219 (2,062–2,388) | −4 | 1 | −2 | 1 | 2 | 1 |
| IL-10 | 48 h | 193 (180–207) | 181 (166–198) | 201 (181–224) | +6 | 1 | −4 | 1 | −10 | 1 |
| IL-6 | 24 h | 6,590 (5,962–7,284) | 5,610 (4,641–6,782) | 5,283 (4,373–6,383) | +17 | 1 | +25 | 0.7859 | +6 | 1 |
| IL-17 | 7 days | 160 (137–186) | 106 (83–136) | 103 (88–122) | +51 | 0.7123 | +54 | <0.0001 | +2 | 1 |
| IL-22 | 7 days | 662 (618–711) | 1,061 (914–1,231) | 963 (860–1,079) | −38 | 0.0002 | −31 | <0.0001 | +10 | 1 |
Data were calculated by multivariate regression on ln-transformed data. The outcomes were transformed back from ln-transformed data to show predicted means in pg/ml. The Bonferroni correction was applied to the P values to account for the multiple comparisons made. 95% CI, 95% confidence interval.
Fig 4.
IL-1β responses of 10 healthy donors to modern and ancient Beijing strains and Euro-American reactivation strains. (A) Strain-associated differences in cytokine production. (B) Donor-associated differences in cytokine production for modern and ancient Beijing strains, for all 10 donors individually.
Modern Beijing strains also induced less production of IFN-γ (at 48 h) than that of the more ancient Beijing strains (48 versus 80 pg/ml) (P = 0.002). TNF-α production at 24 h was slightly lower for modern than for ancient Beijing strains (300 and 365 pg/ml) (nonsignificant difference), and interestingly, both groups induced much less TNF-α than the Euro-American reactivation strains did (623 pg/ml) (nonsignificant difference for ancient Beijing strains after the Bonferroni correction; P < 0.0001 for modern Beijing strains). Production of IL-22 at 7 days was significantly lower in modern Beijing strains than in ancient Beijing strains (662 versus 1,061 pg/ml) (P = 0.0002). Conversely, induction of IL-17 at 7 days was higher in modern Beijing strains than in ancient Beijing strains (160 pg/ml versus 106 pg/ml) (nonsignificant difference) and the reactivation strains (103 pg/ml) (P < 0.0001) (Fig. 3B). No significant differences were found for production of IL-6 (24 h), TGF-β (24 h), and IL-10 (48 h).
DISCUSSION
In our in vitro model, heat-killed and bead-disrupted lysates of modern (“typical”) M. tuberculosis Beijing strains induced a clearly different cytokine signature in freshly isolated PBMCs from that of ancient (“atypical”) Beijing strains. Overall, modern Beijing strains induced considerably less production of IL-1β, IFN-γ, and IL-22 and moderately more production of IL-1Ra and IL-17. Interestingly, stimulation with Euro-American reactivation strains from elderly patients resembled stimulation with ancient Beijing strains, except for the case of TNF-α. Euro-American reactivation strains induced 2-fold more TNF-α than both types of Beijing strains did, which may represent a possible explanation for why infections with these strains appear in such patients only under circumstances of waning immunity.
We hypothesize that the lower levels of induction of proinflammatory cytokines may help to explain the increased spread of modern Beijing strains across the globe. The presence of an IS6110 element in the NTF chromosomal region is traditionally used to distinguish modern from ancient Beijing strains. Deletions that occurred after the insertion of IS6110 are used to further type the group of modern Beijing strains, but so far, there are insufficient epidemiological data to define a genetic subgroup within the modern Beijing strains that is driving their success. For this reason, we decided to compare the group of modern Beijing strains as a whole to the other groups.
A striking observation in the present data is that the ancient Beijing strains, which are considered genetically heterogeneous (6), showed rather uniform cytokine responses, and also that only four of seven genetically modern Beijing genotypes showed the distinctive low inflammatory response. The modern strains that showed the least induction of proinflammatory cytokines may share specific properties that enable them to counteract or subvert effective host responses, and one could thus hypothesize that an even narrower subgroup within the modern Beijing strains is in fact responsible for their global emergence. In future epidemiological and experimental studies, mutations and deletions that occurred subsequent to the insertion of IS6110 into the NTF region in the evolution of modern Beijing strains will have to be assessed to define this group more specifically.
The most striking difference in our study was the almost 2-fold less IL-1β production induced in PBMCs by modern Beijing strains than by ancient Beijing strains. For IL-1Ra, the opposite was found, further limiting the activity of IL-1β after stimulation with modern Beijing strains. IL-1β is increasingly recognized as an important cytokine involved in host defense against M. tuberculosis. IL-1β restricts mycobacterial growth in murine models, and IL-1β knockout mice are highly susceptible to mycobacteria. In humans, polymorphisms in IL-1β or IL-1R are associated with increased tuberculosis susceptibility and progression (19).
Modern M. tuberculosis Beijing strains also induced less production of IFN-γ, which has a well-established role in protection against mycobacterial infections, including tuberculosis (20, 21). Apart from lymphocytes, innate immune cells (NK cells, NK-T cells, and γδ T cells) also contribute to the production of IFN-γ in response to mycobacteria (19, 22). TNF-α production was strikingly lower in response to both types of Beijing strains than in response to the Euro-American strains isolated from patients with reactivation tuberculosis. This is of specific interest because of the swift reactivation of tuberculosis after TNF-α-blocking therapy (23). TNF-α has a paramount role in granuloma formation and maintenance (24) and contributes importantly to the balance of pro- and anti-inflammatory cytokines that determines the success of mycobacterial control (25).
IL-17, which was induced in larger amounts by modern Beijing strains, especially compared to Euro-American reactivation strains, may act as a double-edged sword: it facilitates the formation of mature granulomas, but in excess it leads to enhanced neutrophil recruitment and concurrent lung tissue damage (26). Interestingly, a zebrafish model showed that in the early phase of granuloma formation, IL-17 also facilitates bacterial spread (27), which makes a high IL-17 concentration possibly favorable to bacteria (22). In contrast, IL-22 production was lower in modern Beijing strains. IL-22 is produced in the lung and has been found in bronchoalveolar lavage fluid (28). In general, it provides cross talk between immune cells that produce IL-22 but lack the receptor and nonimmune cells, e.g., lung epithelium cells, that do express the IL-22 receptor. Activation of the IL-22 receptor on these cells leads to upregulation of several chemokines in the lung (29). Lower levels of IL-22, like those found in modern Beijing strains, might thus lead to less expression of chemokines, possibly favoring outgrowth of M. tuberculosis. However, a definitive role of IL-22 in human tuberculosis still has to be confirmed: in a mouse model of tuberculosis, the neutralization of IL-22 did not increase the bacterial burden in the lungs (26).
In recent years, a number of studies have examined whether M. tuberculosis strains from different genetic backgrounds induce strain-specific differences in cytokine production. In the global phylogeny of M. tuberculosis, evolutionarily modern strains—bearing the TbD1 deletion, as all Euro-American and Beijing strains do—generally have a tendency toward inducing a smaller cytokine response, as recently shown comprehensively in a macrophage infection model by Portevin et al. (30). Other studies, using small numbers of strains from a wide range of lineages, have shown the existence of clear but ill-reproducible differences (extensively reviewed by Coscolla and Gagneux [31]). However, it is likely that most of the evolution of M. tuberculosis occurs within its main lineages, and our study is the first with a comprehensive assessment of immune-stimulatory capacity of intralineage strains. These lineages show a distinctive geographical pattern (32) and may adapt to and shape the specific host populations they encounter.
Few studies have examined cytokine production in multiple strains within one of the most successful lineages of M. tuberculosis, the Beijing genotype. Wang et al. found less TNF-α induction for Beijing strains than for H37Rv, with a trend toward lower TNF-α concentrations after stimulation with two ancient Beijing strains (33). In two macrophage infection models, one Beijing isolate appeared to be more immunogenic than H37Rv, with more mRNA expression for IL-1β, TNF-α (34), and IFN-γ (35). Krishnan et al. found less TNF-α induction with the Beijing outbreak strain HN878 but variable results for other Beijing strains (36). Kato-Maeda et al. compared Beijing strains with different abilities to cause secondary disease in humans for their pathogenicity in guinea pigs. Strains were characterized using genetic regions of difference. Interestingly, guinea pigs appeared to be most susceptible to ancient Beijing strains (RD207−). In line with our study, the authors of that study found that strains in the modern sublineages RD142− and RD150− induced less TNF-α mRNA. Expression of IFN-γ mRNA varied among the modern sublineages, and IL-1β was not measured (37).
To our knowledge, the present study is the first that specifically examines the successful subbranch of modern strains within M. tuberculosis Beijing strains. There are, however, a few limitations to this study. To maximize standardization, we used a model in which freshly isolated PBMCs were stimulated with heat-killed and bead-disrupted lysates. Although these lysates, stored in many similar aliquots, produce highly reproducible cytokine patterns, they of course lack some of the lipid structures present in the M. tuberculosis cell wall. In addition, the use of PBMCs may not fully reflect the response of resident tissue macrophages, although it is not logical to expect that the differences in cytokine profiles we found would be reversed in macrophages, which are related to circulating monocytes. An alternative approach would have been to infect macrophages with live mycobacteria. This interesting model has the disadvantages of less reproducibility and an absence of lymphocytes. Also, differences in growth kinetics or mycobacterial gene expression under specific conditions may themselves lead to differences in cytokine induction. Yet another model would be to measure circulating cytokines in patients infected with different genotype strains, though the possibilities are limited by differences in disease status; the large variety of genotype groups, which hinders sound statistical analysis; and the fact that plasma cytokine concentrations are generally low in tuberculosis patients. An Ethiopian study designed this way hinted at less induction of IL-4 in patients infected with Euro-American strains than in those infected with East African-Indian strains (38).
As a strong point, our study design and approach to analyze the results are thorough and innovative. We used 10 donors and 20 different strains belonging to three subgroups (modern and ancient Beijing strains as well as tuberculosis reactivation strains). This approach provided enough power to detect meaningful differences despite variation between donors and different strains within one subgroup, as was clearly the case for modern Beijing strains. Hierarchical clustering, which so far has been used mostly for gene expression data sets, was used to analyze cytokine signatures. Radar graphs were used to graphically represent cytokine-specific differences between the three groups of M. tuberculosis strains.
We conclude that modern Beijing strains show less induction of IL-1β, IFN-γ, and IL-22 and more induction of IL-1Ra in vitro compared to ancient Beijing strains and Euro-American reactivation strains. This differential immune induction might contribute to the epidemiological success of modern M. tuberculosis Beijing strains.
Supplementary Material
ACKNOWLEDGMENTS
We thank Cor Jacobs, Jessica de Beer, Tridia van der Laan, Arnout Mulder, and Louis Wilson for technical help and Jochem Dijkstra for graphical design of the radar graphs. We are most grateful to all collaborators worldwide who provided strains for typing, including Margaret Fitzgibbon of the Irish Mycobacteria Reference Laboratory, St. James's Hospital, Dublin, Ireland, and Maryse Fauville-Dufaux of Tuberculosis and Mycobacteria, Belgian Scientific Institute for Public Health, Brussels, Belgium.
We do not have any commercial or other association that might pose a conflict of interest.
This work was supported by the European Commission (NEWTBVAC grant 41745 and ADITEC grant 280873 to T.H.M.O.), the Netherlands Leprosy Relief Foundation (grant 702.02.72 to T.H.M.O.), and The Netherlands Organisation for Scientific Research (Vidi grant 017.106.310 to R.V.C. and Vici grant 918.10.610 to M.G.N.).
Footnotes
Published ahead of print 29 July 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/IAI.00282-13.
REFERENCES
- 1.van Soolingen D, Qian L, de Haas PE, Douglas JT, Traore H, Portaels F, Qing HZ, Enkhsaikan D, Nymadawa P, van Embden JD. 1995. Predominance of a single genotype of Mycobacterium tuberculosis in countries of East Asia. J. Clin. Microbiol. 33:3234–3238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hershberg R, Lipatov M, Small PM, Sheffer H, Niemann S, Homolka S, Roach JC, Kremer K, Petrov DA, Feldman MW, Gagneux S. 2008. High functional diversity in Mycobacterium tuberculosis driven by genetic drift and human demography. PLoS Biol. 6:e311. 10.1371/journal.pbio.0060311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Parwati I, van Crevel R, van Soolingen D. 2010. Possible underlying mechanisms for successful emergence of the Mycobacterium tuberculosis Beijing genotype strains. Lancet Infect. Dis. 10:103–111 [DOI] [PubMed] [Google Scholar]
- 4.Cobelens F. 2012. Relative reproductive fitness of the W-Beijing genotype. Int. J. Tuberc. Lung Dis. 16:287. [DOI] [PubMed] [Google Scholar]
- 5.Mokrousov I, Narvskaya O, Otten T, Vyazovaya A, Limeschenko E, Steklova L, Vyshnevskyi B. 2002. Phylogenetic reconstruction within Mycobacterium tuberculosis Beijing genotype in northwestern Russia. Res. Microbiol. 153:629–637 [DOI] [PubMed] [Google Scholar]
- 6.Schürch AC, Kremer K, Warren RM, Hung NV, Zhao Y, Wan K, Boeree MJ, Siezen RJ, Smith NH, van Soolingen D. 2011. Mutations in the regulatory network underlie the recent clonal expansion of a dominant subclone of the Mycobacterium tuberculosis Beijing genotype. Infect. Genet. Evol. 11:587–597 [DOI] [PubMed] [Google Scholar]
- 7.Kremer K. 2009. Vaccine-induced immunity circumvented by typical Mycobacterium tuberculosis Beijing strains. Emerg. Infect. Dis. 15:335–339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mokrousov I, Jiao WW, Valcheva V, Vyazovaya A, Otten T, Ly HM, Lan NN, Limeschenko E, Markova N, Vyshnevskiy B, Shen AD, Narvskaya O. 2006. Rapid detection of the Mycobacterium tuberculosis Beijing genotype and its ancient and modern sublineages by IS6110-based inverse PCR. J. Clin. Microbiol. 44:2851–2856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dou H-Y, Tseng F-C, Lin C-W, Chang J-R, Sun J-R, Tsai W-S, Lee S-Y, Su I-J, Lu J-J. 2008. Molecular epidemiology and evolutionary genetics of Mycobacterium tuberculosis in Taipei. BMC Infect. Dis. 8:170. 10.1186/1471-2334-8-170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Strauss OJ, Warren RM, Jordaan A, Streicher EM, Hanekom M, Falmer AA, Albert H, Trollip A, Hoosain E, van Helden PD, Victor TC. 2008. Spread of a low-fitness drug-resistant Mycobacterium tuberculosis strain in a setting of high human immunodeficiency virus prevalence. J. Clin. Microbiol. 46:1514–1516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kato-Maeda M, Kim EY, Flores L, Jarlsberg LG, Osmond D, Hopewell PC. 2010. Differences among sublineages of the East-Asian lineage of Mycobacterium tuberculosis in genotypic clustering. Int. J. Tuberc. Lung Dis. 14:538–544 [PMC free article] [PubMed] [Google Scholar]
- 12.Iwamoto T, Fujiyama R, Yoshida S, Wada T, Shirai C, Kawakami Y. 2009. Population structure dynamics of Mycobacterium tuberculosis Beijing strains during past decades in Japan. J. Clin. Microbiol. 47:3340–3343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wada T, Fujihara S, Shimouchi A, Harada M, Ogura H, Matsumoto S, Hase A. 2009. High transmissibility of the modern Beijing Mycobacterium tuberculosis in homeless patients of Japan. Tuberculosis 89:252–255 [DOI] [PubMed] [Google Scholar]
- 14.Ioerger TR, Feng Y, Chen X, Dobos KM, Victor TC, Streicher EM, Warren RM, van Pittius NCG, van Helden PD, Sacchettini JC. 2010. The non-clonality of drug resistance in Beijing-genotype isolates of Mycobacterium tuberculosis from the Western Cape of South Africa. BMC Genomics 11:670. 10.1186/1471-2164-11-670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mokrousov I, Jiao W-W, Sun G-Z, Liu J-W, Valcheva V, Li M, Narvskaya O, Shen A-D. 2006. Evolution of drug resistance in different sublineages of Mycobacterium tuberculosis Beijing genotype. Antimicrob. Agents Chemother. 50:2820–2823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jiao W-W, Mokrousov I, Sun G-Z, Li M, Liu J-W, Narvskaya O, Shen A-D. 2007. Molecular characteristics of rifampin and isoniazid resistant Mycobacterium tuberculosis strains from Beijing, China. Chin. Med. J. 120:814–819 [PubMed] [Google Scholar]
- 17.Plikaytis BB, Marden JL, Crawford JT, Woodley CL, Butler WR, Shinnick TM. 1994. Multiplex PCR assay specific for the multidrug-resistant strain W of Mycobacterium tuberculosis. J. Clin. Microbiol. 32:1542–1546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dysvik B, Jonassen I. 2001. J-Express: exploring gene expression data using Java. Bioinformatics 17:369–370 [DOI] [PubMed] [Google Scholar]
- 19.Cooper AM, Mayer-Barber KD, Sher A. 2011. Role of innate cytokines in mycobacterial infection. Mucosal Immunol. 4:252–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Crevel R, Ottenhoff THM, van der Meer JWM. 2002. Innate immunity to Mycobacterium tuberculosis. Clin. Microbiol. Rev. 15:294–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ottenhoff THM, Verreck FAW, Lichtenauer-Kaligis EGR, Hoeve MA, Sanal O, van Dissel JT. 2002. Genetics, cytokines and human infectious disease: lessons from weakly pathogenic mycobacteria and salmonellae. Nat. Genet. 32:97–105 [DOI] [PubMed] [Google Scholar]
- 22.Ottenhoff THM. 2012. New pathways of protective and pathological host defense to mycobacteria. Trends Microbiol. 20:419–428 [DOI] [PubMed] [Google Scholar]
- 23.Keane J, Gershon S, Wise RP, Mirabile-Levens E, Kasznica J, Schwieterman WD, Siegel JN, Braun MM. 2001. Tuberculosis associated with infliximab, a tumor necrosis factor alpha-neutralizing agent. N. Engl. J. Med. 345:1098–1104 [DOI] [PubMed] [Google Scholar]
- 24.Miller EA, Ernst JD. 2008. Illuminating the black box of TNF action in tuberculous granulomas. Immunity 29:175–177 [DOI] [PubMed] [Google Scholar]
- 25.Flynn JL, Chan J, Lin PL. 2011. Macrophages and control of granulomatous inflammation in tuberculosis. Mucosal Immunol. 4:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Torrado ED, Cooper AM. 2010. IL-17 and Th17 cells in tuberculosis. Cytokine Growth Factor Rev. 21:455–462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Davis JM, Ramakrishnan L. 2009. The role of the granuloma in expansion and dissemination of early tuberculous infection. Cell 136:37–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glader P, Smith ME, Malmhäll C, Balder B, Sjöstrand M, Qvarfordt I, Lindén A. 2010. Interleukin-17-producing T-helper cells and related cytokines in human airways exposed to endotoxin. Eur. Respir. J. 36:1155–1164 [DOI] [PubMed] [Google Scholar]
- 29.Sonnenberg GF, Fouser LA, Artis D. 2011. Border patrol: regulation of immunity, inflammation and tissue homeostasis at barrier surfaces by IL-22. Nat. Immunol. 12:383–390 [DOI] [PubMed] [Google Scholar]
- 30.Portevin D, Gagneux S, Comas I, Young D. 2011. Human macrophage responses to clinical isolates from the Mycobacterium tuberculosis complex discriminate between ancient and modern lineages. PLoS Pathog. 7:e1001307. 10.1371/journal.ppat.1001307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Coscolla M, Gagneux S. 2010. Does M. tuberculosis genomic diversity explain disease diversity? Drug Discov. Today Dis. Mech. 7:e43–e59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gagneux S, Small PM. 2007. Global phylogeography of Mycobacterium tuberculosis and implications for tuberculosis product development. Lancet Infect. Dis. 7:328–337 [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Peyron P, Mestre O, Kaplan G, van Soolingen D, Gao Q, Gicquel B, Neyrolles O. 2010. Innate immune response to Mycobacterium tuberculosis Beijing and other genotypes. PLoS One 5:e13594. 10.1371/journal.pone.0013594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chacón-Salinas R, Serafín-López J, Ramos-Payán R, Méndez-Aragón P, Hernandez-Pando R, van Soolingen D, Flores-Romo L, Estrada-Parra S, Estrada-García I. 2005. Differential pattern of cytokine expression by macrophages infected in vitro with different Mycobacterium tuberculosis genotypes. Clin. Exp. Immunol. 140:443–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cappelli G, Volpe P, Sanduzzi A, Sacchi A, Colizzi V, Mariani F. 2001. Human macrophage gamma interferon decreases gene expression but not replication of Mycobacterium tuberculosis: analysis of the host-pathogen reciprocal influence on transcription in a comparison of strains H37Rv and CMT97. Infect. Immun. 69:7262–7270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Krishnan N, Malaga W, Constant P, Caws M, Thi Hoang Chau T, Salmons J, Thi Ngoc Lan N, Bang ND, Daffé M, Young DB, Robertson BD, Guilhot C, Thwaites GE. 2011. Mycobacterium tuberculosis lineage influences innate immune response and virulence and is associated with distinct cell envelope lipid profiles. PLoS One 6:e23870. 10.1371/journal.pone.0023870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kato-Maeda M, Shanley CA, Ackart D, Jarlsberg LG, Shang S, Obregon-Henao A, Harton M, Basaraba RJ, Henao-Tamayo M, Barrozo JC, Rose J, Kawamura LM, Coscolla M, Fofanov VY, Koshinsky H, Gagneux S, Hopewell PC, Ordway DJ, Orme IM. 2012. Beijing sublineages of Mycobacterium tuberculosis differ in pathogenicity in the guinea pig. Clin. Vaccine Immunol. 19:1227–1237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mihret A, Bekele Y, Loxton AG, Aseffa A, Howe R, Walzl G. 2012. Plasma level of IL-4 differs in patients infected with different modern lineages of M. tuberculosis. J. Trop. Med. 2012:518564. 10.1155/2012/518564 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.