Fig 3.
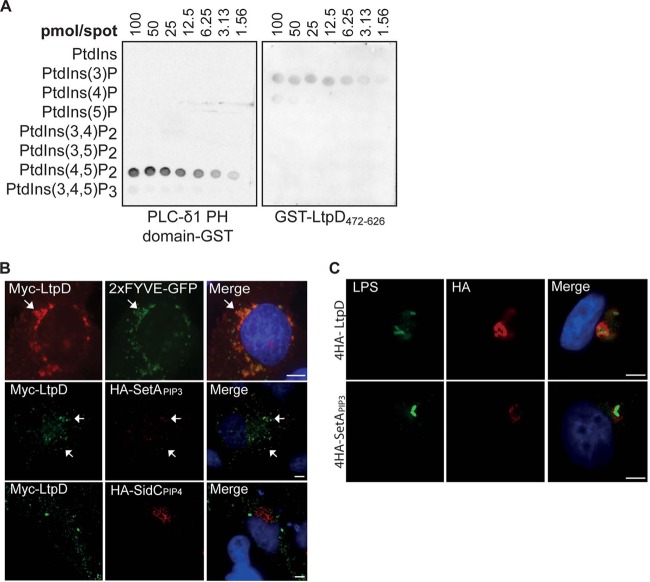
GST-LtpD472-626 binds to PtdIns(3)P in vitro and localizes to PtdIns(3)P-containing membranes. (A) Protein-lipid overlay. Purified GST-LtpD472-626, GST, or a GST fusion of the PH domain of PLC-δ1 was incubated with membranes on which serial dilutions of various PtdIns species were spotted, as indicated. Protein binding to lipids was detected using an anti-GST antibody. LtpD472-626-GST bound preferentially to PtdIns(3)P and, to a smaller extent, to PtdIns(4)P. The PH domain of PLC-δ1 was used as a positive control and bound strongly to PtdIns(3,4)P2. The results are representative of three separate experiments. (B) Upon cotransfection of HeLa cells, Myc-LtpD colocalized with 2×FYVE-GFP and 4HA-SetAPIP3, reporters for PtdIns(3)P, but not HA-SidCPIP4, a marker for PtdIns(4)P. Scale bars, 10 μm. (C) Upon infection of A549 cells with either L. pneumophila 4HA-4LtpD or 4HA-SetAPIP3, 4HA-SetAPIP3 localizes to the LCV, indicating the presence of PtdIns(3)P in the LCV membrane. Scale bars, 5 μm.