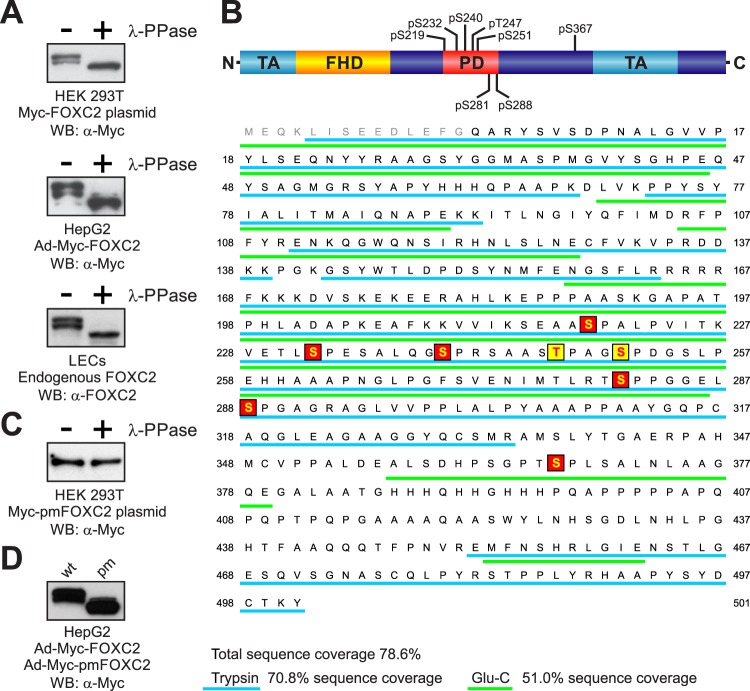
Fig 1.
Analysis of FOXC2 phosphorylation. (A) Endogenous and recombinant human FOXC2 are similarly phosphorylated in primary LECs and immortalized cell lines. Cell lysates were treated (+) or not treated (−) with lambda protein phosphatase (λ-PPase) and analyzed by Western blotting with anti-FOXC2 or anti-Myc antibodies. (B) Schematic representation of FOXC2 phosphorylation sites. FHD, forkhead domain; TA, transactivation domains (5, 34); PD, phosphorylated domain. Phosphorylation sites identified by LC-MS/MS are shaded in red; phosphorylation sites identified by mutagenesis are shaded in yellow. Peptides detected by MS in tryptic and Glu-C digests are underlined in cyan and green, respectively. Amino acid numbering is the same as in the endogenous protein (NP_005242). (C) Substitution of eight phosphorylation sites in Myc-FOXC2 with alanine abolishes the phosphorylation-dependent electrophoretic mobility shift. Lysates of cells transfected with a plasmid expressing the phosphorylation-deficient mutant Myc-pmFOXC2 were treated (+) or not treated (−) with λ-PPase and analyzed by Western blotting with anti-Myc antibody. (D) FOXC2 phosphorylation-deficient mutant (pm) has increased electrophoretic mobility compared to the wild-type (wt) protein. Shown is Western blot analysis of lysates from HepG2 cells transduced with adenoviruses expressing Myc-FOXC2 or Myc-pmFOXC2.