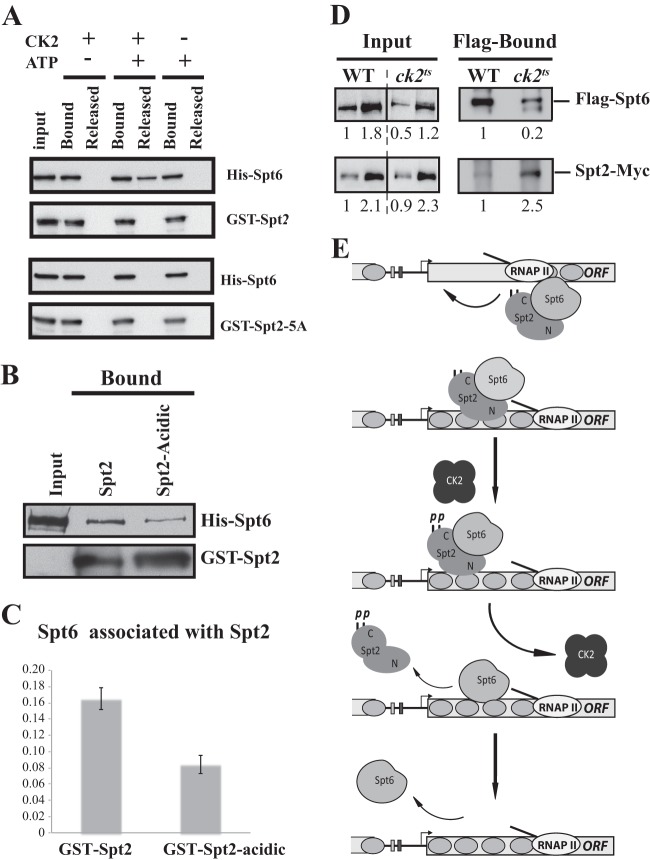
Fig 10.
CK2-dependent phosphorylation of Spt2 RI/RII sites regulates Spt2-Spt6 interactions in vitro and in vivo. (A) Mutation of RI/RII sites to alanine abolishes the disruption of the Spt2-Spt6 complex by CK2 phosphorylation. The His-Spt6/GST-Spt2 or GST–Spt2-5A complex was bound on glutathione beads. After washes, the complex was incubated with or without purified yeast CK2 in presence or absence of ATP. The Spt6 released was assessed in the soluble fraction. As described in the legend of Fig. 9C, proteins were analyzed by anti-His and anti-GST Western blotting. (B) Mutation of RI/RII sites to acidic amino acids decreases the interaction between Spt2 and Spt6. The His-Spt6/GST-Spt2 or GST–Spt2-acidic complex was bound on glutathione beads. After washes, the level of each protein was assessed by anti-His and anti-GST Western blotting. (C) Quantification of the amount of Spt6 that is pulled down by GST-Spt2 and the GST–Spt2-acidic version. The values on the histogram represent the averages and standard errors of two independent experiments. The Western blots were quantified by using a Fluor-S-MultiImager. (D) Depletion of CK2 activity increases the level of Spt2 that interacts with Spt6 in vivo. Shown are coimmunoprecipitation experiments using WCEs of wild-type or ck2ts cells grown to mid-log phase and shifted to a nonpermissive temperature for 2 h. These cells express Flag-Spt6 and Spt2-13Myc, and immunoprecipitation was conducted with Flag antibodies coupled to agarose beads. The immunoprecipitated proteins were analyzed by anti-Flag and anti-Myc Western blotting. The relative amount of each protein was quantified and is reported. (E) Model of Spt2 regulation by CK2-dependent phosphorylation.