Fig 3.
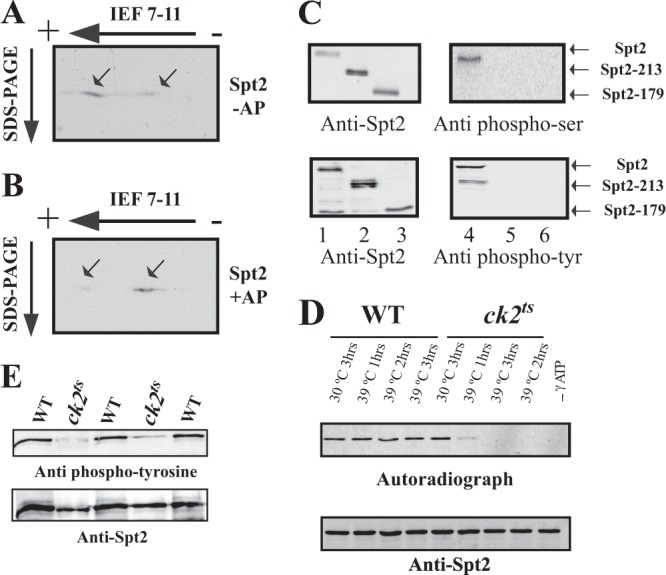
CK2 is required for the in vivo phosphorylation of Spt2. (A and B) Identification of Spt2 isoforms by 2D gel electrophoresis. WCE from a strain expressing Spt2-Flag was subjected (+AP) (B) or not subjected (−AP) (A) to alkaline phosphatase treatment followed by 2D gel electrophoresis (isoelectric focusing [IEF] followed by SDS-PAGE) and then analyzed by anti-Flag Western blotting. The arrows indicate the major Spt2 isoforms. Panel B shows that, after alkaline phosphatase, the migration of the most acidic form of Spt2 is shifted to that of a less acidic isoform(s). (C) Spt2 is phosphorylated in vivo on serine and tyrosine residues located in the C-terminal domain. In vivo phosphorylation of Spt2 domains was assessed by Western blotting using antiphosphoserine (top right), antiphosphotyrosine (bottom right), and anti-Flag (left) antibodies. The WCEs tested were prepared from yeast cells expressing the indicated Spt2-Flag version, subjected to anti-Flag immunoprecipitation, and then analyzed by Western blotting with the indicated antibody. (D) CK2 depletion affects the phosphorylation of Spt2. WCEs were prepared from wild-type or ck2ts cells expressing Spt2-13Myc. These cells were grown at the permissive (30°C) or restrictive (39°C) temperature for the indicated times. Equal amount of WCEs were incubated with radiolabeled ATP. The proteins were immunoprecipitated with anti-Myc antibodies and resolved by SDS-PAGE followed by transfer onto a nitrocellulose membrane for anti-Myc Western blotting (bottom) or autoradiography (top). (E) Spt2 tyrosine phosphorylation is dependent on CK2. In vivo phosphorylation of Spt2 was assessed by antiphosphotyrosine (top) and anti-Myc (bottom) Western blotting. The cells from wild-type or ck2ts strains expressing Spt2-13Myc were grown to mid-log phase at the permissive temperature (30°C) and then shifted to the nonpermissive temperature (37°C) for 2 h. The WCEs from these cells were subjected to anti-Myc immunoprecipitation and then analyzed by Western blotting with the indicated antibody. Three wild-type and two ck2ts mutant clones were analyzed.
