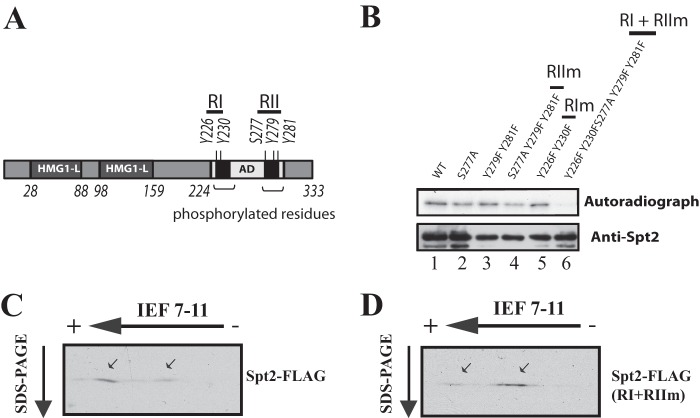
Fig 4.
CK2 phosphorylates five residues on two short regions (RI and RII) in the C-terminal domain of Spt2. (A) Diagram showing the potential CK2 phosphorylation sites on Spt2. (B) Mutations of the RI and RII sites abolish Spt2 phosphorylation by CK2. Kinase assays were performed by using equal amounts of recombinant wild-type His-Spt2 or the indicated mutated version. The samples were analyzed by antihistidine Western blotting (bottom) and autoradiography (top). (C and D) Mutations of RI and RII affect the in vivo phosphorylation of Spt2. Shown are data for 2D-PAGE analysis of Spt2 (wild type [WT]) and Spt2 (RI+RII mutated to alanine). WCEs from a strain expressing Spt2-Flag (C) or a nonphosphorylatable Spt2-Flag version (RI+RII mutated to alanine) (D) were resolved by 2D-PAGE and analyzed by anti-Flag Western blotting.