Fig 9.
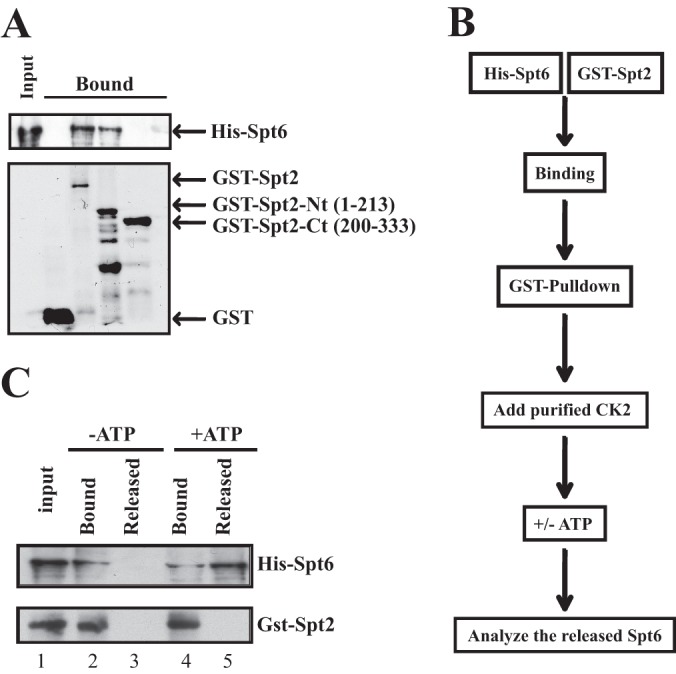
Phosphorylation by CK2 regulates the Spt2-Spt6 interaction. (A) Spt2 interacts directly with Spt6. GST pulldown experiments were conducted by using recombinant GST, GST-Spt2, GST–Spt2-Nt (aa 1 to 213), GST–Spt2-Ct (aa 200 to 333), and His-Spt6. After washes, input (20%) and bound proteins were analyzed by anti-His Western blotting or by Coomassie staining. (B) Diagram summarizing the experimental procedure. (C) The Spt2-Spt6 interaction is regulated by CK2 phosphorylation. The His-Spt6/GST-Spt2 complex was bound on glutathione beads. After washes, the complex was incubated with purified yeast CK2 in the presence of ATP (+ATP) or in the absence of ATP (−ATP). The Spt6 released was assessed in the soluble fraction. The input (15%), the bound fraction (15%), and a large fraction of the supernatant were analyzed by anti-His and anti-GST Western blotting.
