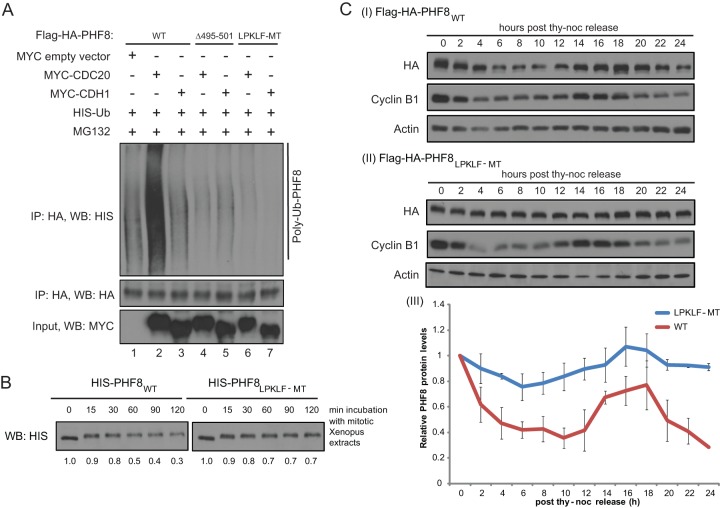
Fig 5.
The PHF8 LPKLF mutant resists polyubiquitylation by the APCcdc20 and proteasomal degradation but has an as-yet-undefined effect on cells. (A) WT or mutant Flag-HA-PHF8 with either the entire 7-amino-acid motif deleted (Δ495–501) or each key amino acid mutated to alanine (LPKLF-MT) was transfected into HEK293T cells together with HIS-Ub and the empty vector, MYC-CDC20, or MYC-CDH1. Forty-eight hours later, cells were treated with MG132 for 4 h and harvested. Equal amounts of denatured lysates were used for HA IP, and immunocomplexes were probed for HIS-Ub. (B) Sf9-purified full-length HIS-tagged WT or LPKLF-MT PHF8 protein was incubated with mitotic Xenopus extracts and harvested at 0, 15, 30, 60, 90, and 120 min. Samples were immunoblotted for HIS. Bands were quantitated by ImageJ and normalized against the sample from 0 min and are presented below the blot. (C) Stable HeLa cells expressing Flag-HA-tagged PHF8WT (I) or PHF8LPKLF-MT (II) were synchronized at M phase and harvested at 2-h intervals upon release for 24 h. Total cell lysates were subjected to immunoblotting. HA (PHF8) levels were quantitated by ImageJ from duplicate experiments and plotted on a graph (III).