Abstract
Objective:
To determine arterial stiffness and β-amyloid (Aβ) deposition in the brain of dementia-free older adults.
Methods:
We studied a cohort of 91 dementia-free participants aged 83–96 years. In 2009, participants completed brain MRI and PET imaging using Pittsburgh compound B (PiB; a marker of amyloid plaques in human brain). In 2011, we measured resting blood pressure (BP), mean arterial pressure (MAP), and arterial stiffness by pulse wave velocity (PWV) in the central, peripheral, and mixed (e.g., brachial ankle PWV [baPWV]) vascular beds, using a noninvasive and automated waveform analyzer.
Results:
A total of 44/91 subjects were Aβ-positive on PET scan. Aβ deposition was associated with mixed PWV, systolic BP, and MAP. One SD increase in baPWV resulted in a 2-fold increase in the odds of being Aβ-positive (p = 0.007). High white matter hyperintensity (WMH) burden was associated with increased central PWV, systolic BP, and MAP. Compared to Aβ-negative individuals with low WMH burden, each SD increase in PWV was associated with a 2-fold to 4-fold increase in the odds of being Aβ-positive and having high WMH.
Conclusions:
Arterial stiffness was associated with Aβ plaque deposition in the brain, independent of BP and APOE ε4 allele. The associations differed by type of brain abnormality and vascular bed measured (e.g., WMH with central stiffness and Aβ deposition and mixed stiffness). Arterial stiffness was highest in individuals with both high Aβ deposition and WMH, which has been suggested to be a “double hit” contributing to the development of symptomatic dementia.
Hypertension is linked to cognitive impairment and the pathologic features of Alzheimer disease (AD), including neurofibrillary tangles and β-amyloid (Aβ) plaques,1 as well as small-vessel disease and white matter hyperintensities (WMH) in the brain.2 Arterial stiffness appears to play a major role in the relationship between hypertension and its consequences in the brain; mounting evidence implicates arterial stiffness in the pathogenesis of impaired cognitive function and dementia in the elderly.3
With the development of in vivo Aβ plaque imaging (e.g., Pittsburgh compound B [PiB]–PET]), we and others have demonstrated that more than half of adults over age 80 without dementia have significant fibrillar Aβ deposition, as indicated by positive PiB scans.4 Except for APOE ε4 genotype and aging, the risk factors and determinants of Aβ deposition in brain are poorly understood.
Recent studies show that blood pressure (BP) is associated with brain Aβ deposition as measured by PiB-PET5,6; thus, arterial stiffness may play a central role in these associations. The risk of clinical AD is higher for Aβ-positive individuals with concomitant subclinical cerebrovascular disease, i.e., WMH.7 Arterial stiffness is a well-established risk factor for subclinical cerebrovascular disease and WMH,8 independent of other cardiovascular risk factors.9,10 Yet the relationship between arterial stiffness and Aβ deposition is unknown. Therefore, in this observational study of very elderly adults without dementia, we evaluated the relationship of arterial stiffness with measures of brain structure, including cerebral fibrillar Aβ deposition and WMH volume, considered separately and jointly.
METHODS
Subjects were recruited from the Ginkgo Evaluation of Memory Study (GEMS, 2000–2008). This was a multisite, placebo-controlled, double-blind, randomized clinical trial of daily use of ginkgo biloba in 3,069 community-dwelling participants aged 72–96 years at baseline.11 In 2009, approximately 10 months following the GEMS drug closeout visit, 194 participants from the Pittsburgh site underwent brain MRI and PiB-PET as part of the GEMS Imaging Sub-Study, detailed in Mathis et al.4 In 2011, approximately 2 years following neuroimaging, 47% (91/194) of these GEMS Imaging Sub-Study participants without dementia returned to the clinic for measures of arterial stiffness.
Standard protocol approvals, registrations, and patient consents.
This study received local institutional review board approval prior to study initiation. All participants completed the informed consent process prior to any study procedures.
PET and MRI of the brain.
Details of PiB-PET data acquisition have been described previously.4 We used the iterative mild outlier cutoff method (standardized uptake value ratio was >1.57)12 to determine Aβ positivity and compared these results to those obtained using the sparse k-means approach13 and found them to be nearly identical. Results from the iterative outlier method are presented herein.
MRI.
MRI scanning utilized a GE Signa 1.5T scanner and standard head coil, as well as MRI processing, using methods described previously.14–16 WMH were visualized from T2-weighted fluid-attenuated inversion recovery images using an automated method for localization of WMH and quantification using a fuzzy connected algorithm with automated seed selection.17 Total WMH volume (WMHv) was estimated by summing all voxels classified as WMH and then normalized by total parenchymal volume brain volume.
Clinical assessments.
Just prior to PET imaging and MRI of the brain, participants underwent cognitive evaluation, 10-question Center for Epidemiologic Studies Depression Scale, timed walk, and inventory of their prescription and over-the-counter medications. Cognitive adjudication was performed blind to neuroimaging results by the Cognitive Diagnostic Center, taking into account historical serial cognitive assessments from the parent GEMS11 as described in detail by Snitz et al.18 Criteria for mild cognitive impairment (MCI) included 1–3 tests impaired at cutoffs of 1.5 SD below age- and education-adjusted means.
Arterial dynamics.
Arterial stiffness was measured by pulse wave velocity (PWV) using a noninvasive and automated waveform analyzer (VP2000, Omron Co., Komaki, Japan).19 All measures were performed under standardized conditions as previously described.20 PWV was measured in the central (carotid-femoral [cfPWV] and heart-femoral [hfPWV]), peripheral (femoral-ankle [faPWV]), and mixed (brachial-ankle [baPWV]) vascular beds. PWV was calculated as the distance in centimeters between arterial sites of interest over time (in seconds) that the pressure waveforms traveled from the heart to the respective arterial sites. Site-specific measurements were detailed previously.20 The average of 2 runs was calculated to determine average PWV. For baPWV, the average PWV of the left and right sides was utilized in the analysis. Validity and reliability of PWV assessment with this device has been reported.21 Reproducibility of PWV measures was determined using intraclass correlation coefficients (ICC). ICC was higher for baPWV (ICC = 0.97) and faPWV (ICC = 0.96) compared to cfPWV (ICC = 0.75).
Potential covariates.
Age, height, and weight were assessed at the same time as arterial measures and used to calculate body mass index (BMI) by standard means. APOE ε4 carrier genotyping and medication assessments were made during the GEMS. Antihypertensive medication use was assessed at each study visit in GEMS between 2000 and 2009.11 For the purpose of this analysis, participants were categorized as ever using antihypertensive medication during the GEMS (2000–2008).
Statistical analysis.
Differences in participant characteristics by Aβ status (Aβ-positive and Aβ-negative) were assessed using logistic regression adjusting for age and sex. The normality of the distributions of continuous measures of brain structure (Aβ deposition, WMHv, and gray matter volume/intracranial volume) and vascular measures were assessed using histograms and univariate statistics. Intercorrelations among normally distributed measures were assessed using Pearson correlation coefficients, and by Spearman correlation coefficients for skewed measures of brain structure. Brain structure outcomes were divided into tertiles for each distribution in order to examine linearity of associations and analysis of covariance was used to calculate means and 95% confidence limits across tertiles of brain outcomes in multivariable models. Multivariable logistic regression models were constructed to determine the odds of being Aβ-positive per 1 SD increase in measures of arterial stiffness and pressure. Multivariable modeling made adjustment for age, sex, BMI, and antihypertensive medication use. Effect modification by sex, cognitive status, and APOE ε4 allele carrier status was assessed by interaction terms within models and also by repeating logistic models after stratification by potential effect modifiers. To assess the relationship of arterial stiffness to both the individual and combined odds of having high Aβ and high WMH burden, a 4-level composite variable was created that combined Aβ status and WMH burden (high vs low) based upon the median split of the continuous WMHv distribution. Multinomial multivariable logistic regression was used to calculate the individual and combined odds of having high WMH and being Aβ-positive relative to the referent group (low WMH and Aβ-negative), adjusted for age, sex, BMI, and antihypertensive medication use.
RESULTS
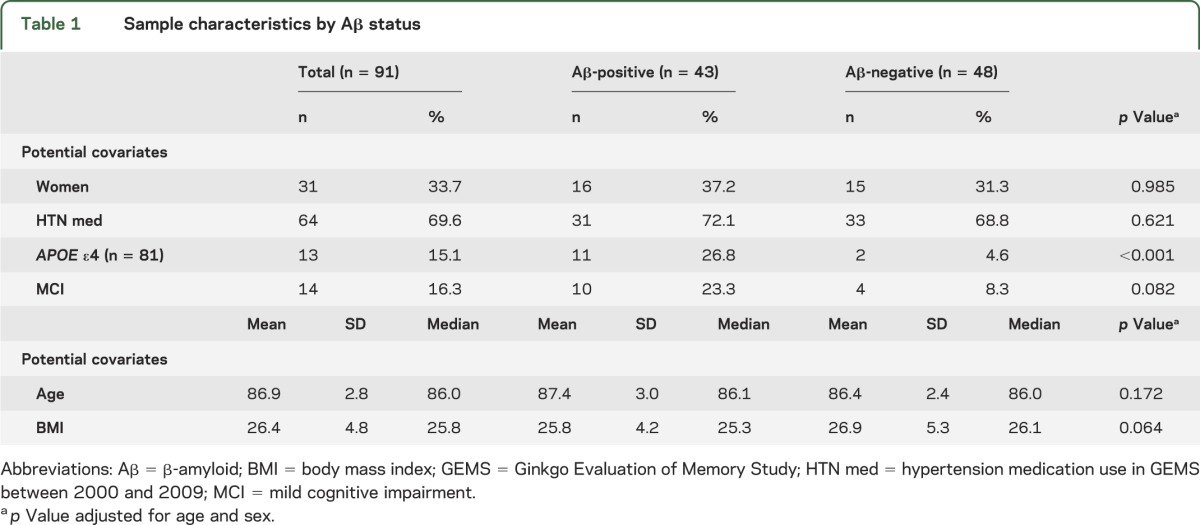
The 91 participants with measures of arterial stiffness and PiB-PET had a mean age of 87 ± 3 years and BMI of 26 ± 5; 33% (n = 31) were women, 15% (n = 13/86) were APOE ε4 carriers, and 15% (n = 14) were classified as MCI at the time of neuroimaging (table 1). More than 70% (n = 64) of participants who had PWV measured were on antihypertensive medications. Compared to the 113 remaining GEMS neuroimaging participants with PiB-PET, participants with arterial stiffness were slightly more likely to be men, to be non-APOE ε4 carriers, to have higher global cognitive scores, and to have lower BP (detailed in table e-1 on the Neurology® Web site at www.neurology.org).
Table 1.
Sample characteristics by Aβ status
Relationship between Aβ deposition and PWV.
The cfPWV, baPWV, hfPWV, and faPWV were highly correlated with each other (Pearson rho = 0.50–0.62, p < 0.05), and did not differ by sex, cognitive status, APOE ε4 carrier status, or antihypertensive medication use. Mean and median levels of baPWV (p < 0.01) and systolic BP (SBP) (p = 0.04) were both higher in Aβ-positive participants (table 2). Other measures of PWV were also higher in the Aβ-positive cases, but were not statistically significant. Neither diastolic BP (DBP) nor pulse rate was associated with Aβ status. The odds of being Aβ-positive nearly doubled for every SD increase in baPWV (odds ratio [OR] [95% confidence interval (CI)] = 1.90 [1.17–3.10]). The odds of being Aβ-positive increased more than 1.5 times for every 1 SD increase in SBP (OR [95% CI] = 1.69 [1.02–2.81]). These associations with Aβ status were independent of age, sex, BMI, and antihypertensive medication use. Despite strong correlations between baPWV and SBP (Spearman rho = 0.52, p < 0.01), including baPWV and SBP in the same model (already adjusted for age, sex, BMI, and antihypertensive medication use) provided modest attenuation of SBP (p = 0.22) and only mild attenuation of baPWV (p = 0.06), suggesting that the effects of PWV remained correlated with but largely independent of BP. The relationship between baPWV was similar for both men and women, separately; however, the association between Aβ status and SBP and hfPWV was stronger in men compared to women (data not shown). The addition of APOE ε4 carrier status and cognitive status individually to each model did not modify these results.
Table 2.
Measures of arterial stiffness by Aβ status
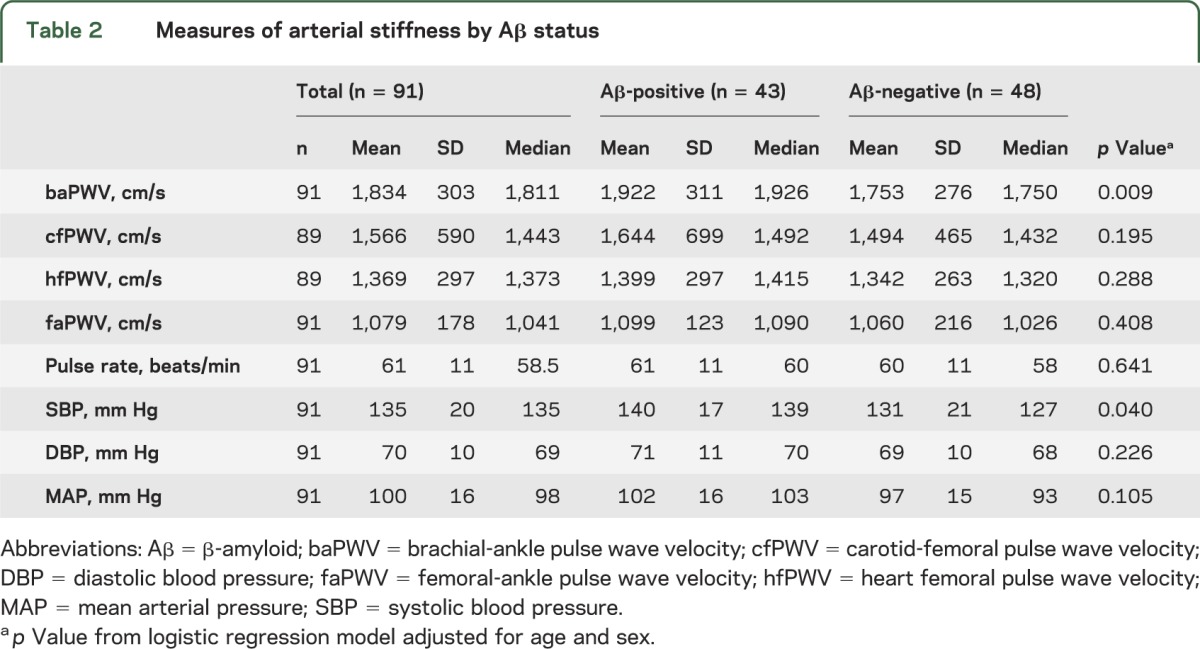
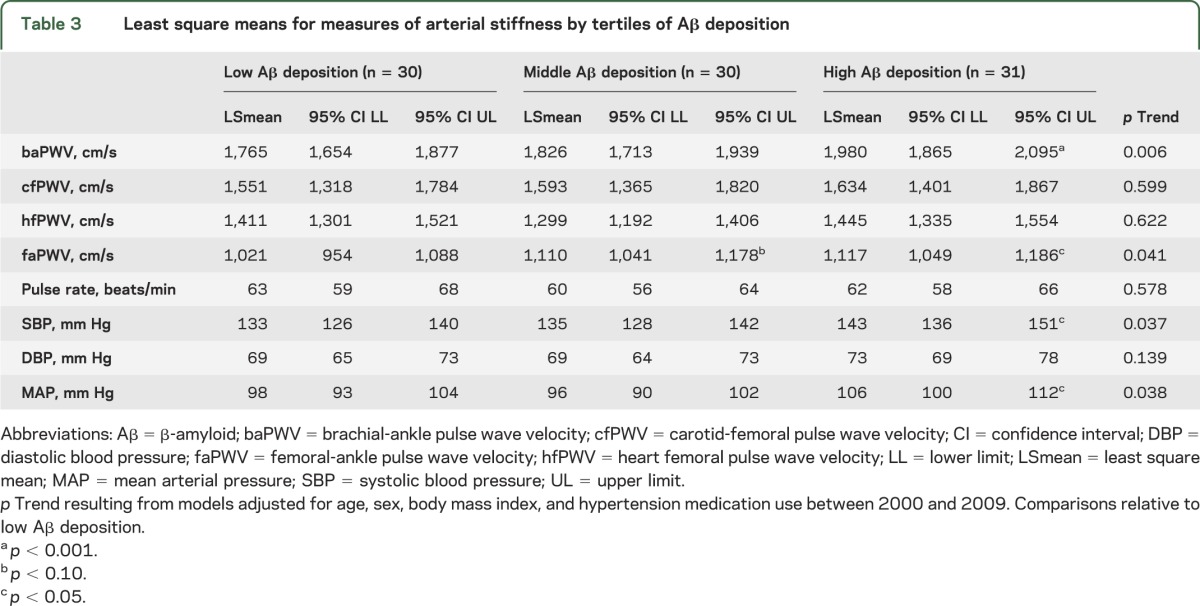
As a continuous measure, Aβ deposition was significantly correlated with baPWV, faPWV, SBP, and mean arterial pressure (MAP) (Spearman rho = 0.22–0.33), but not with central measures of arterial stiffness (cfPWV, p = 0.30; and hfPWV, p = 0.11). Similar results were observed with tertiles of Aβ deposition, where the mean levels of baPWV, faPWV, SBP, and MAP were higher with higher tertiles of Aβ (p < 0.05 for all), adjusted for age, sex, BMI, and antihypertensive medication use (table 3).
Table 3.
Least square means for measures of arterial stiffness by tertiles of Aβ deposition
White matter hyperintensities were associated with central PWV.
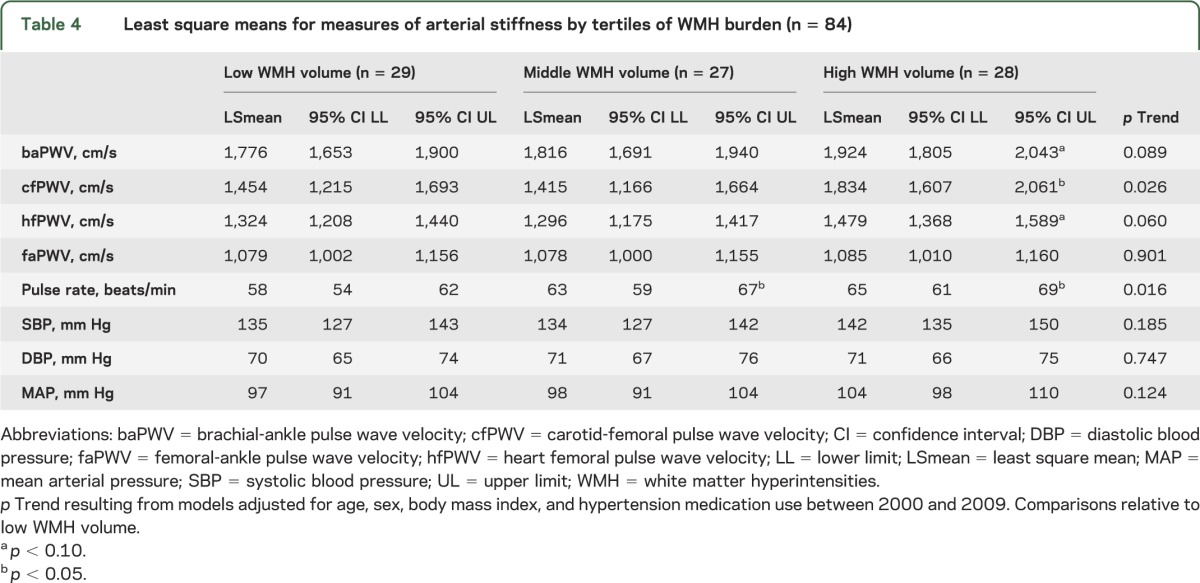
Continuous measures of WMHv were significantly correlated with central measures of arterial stiffness (cfPWV [rho = 0.35, p < 0.01] and hfPWV [rho = 0.26, p = 0.02]), SBP (rho = 0.25, p = 0.02), and MAP (rho = 0.23, p = 0.03), but were not significantly correlated with baPWV or faPWV (p > 0.12). In models adjusted for age, sex, BMI, and use of hypertension medications (table 4), cfPWV (p = 0.03) and pulse rate (p = 0.02) were higher across tertiles of increasing WMHv (p for linear trend <0.05), but the linear trend for baPWV did not reach statistical significance (p = 0.09).
Table 4.
Least square means for measures of arterial stiffness by tertiles of WMH burden (n = 84)
Gray matter volume was not associated with vascular measures.
Continuous measures of gray matter volume were not significantly correlated with arterial pressure or stiffness (all p > 0.24). The same lack of associations with vascular measures and gray matter volume were noted across tertiles of gray matter volume (table e-2).
Arterial stiffness was worse in individuals with both high WMH and high Aβ deposition.
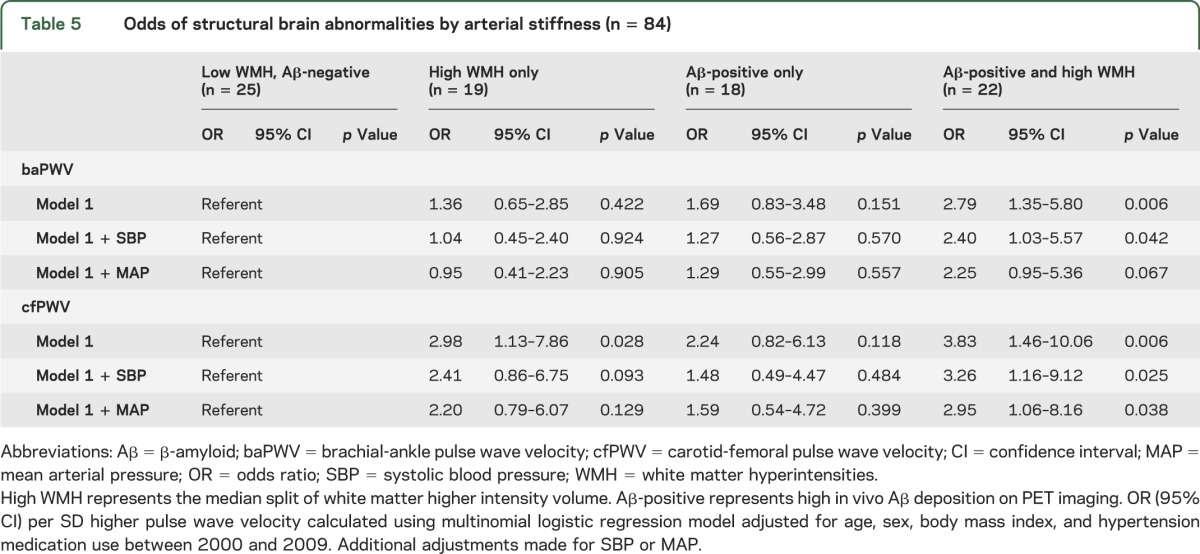
Finally, we evaluated cfPWV and baPWV in relation to the individual and combined outcomes of Aβ status and WMHv. Interestingly, WMHv and Aβ accumulation were not significantly correlated with each other in this sample (log-transformed WMHv and continuous Aβ accumulation, Pearson rho = −0.03, p = 0.76). Compared to Aβ-negative individuals with low WMH, a 1 SD change in baPWV or cfPWV were both significantly associated with a 2-fold to 4-fold increase in the odds of being both Aβ-positive and having high WMH (table 5). These associations held after making additional adjustments for SBP and MAP. The odds of high WMH alone was significantly associated with higher cfPWV, but not baPWV. The odds of being Aβ-positive with low WMH were only slightly higher with increasing baPWV (p = 0.15) and cfPWV (p = 0.11).
Table 5.
Odds of structural brain abnormalities by arterial stiffness (n = 84)
DISCUSSION
Arterial stiffness and BP were associated with severity of Aβ deposition and subclinical small-vessel disease in the brains of very elderly adults without dementia. However, the associations between arterial stiffness and brain structural abnormalities were largely independent of BP and appeared to differ by type of brain abnormality and vascular bed measured. Aβ deposition in the brain (a hallmark of AD) was more strongly associated with mixed measures of arterial stiffness (e.g., baPWV) than central measures, while white matter disease burden was more strongly associated with central measures of arterial stiffness alone. These associations were independent of age, sex, BMI, antihypertensive medication use, and presence of the APOE ε4 allele, and were not modified by sex, cognition, or APOE ε4 carrier status. Only the relationship between Aβ status and hfPWV differed by sex, with stronger associations seen in men than women. While WMH and Aβ deposition were not significantly correlated, co-occurrence of the two showed the strongest relationships with arterial stiffness. Each SD increase in baPWV and cfPWV was associated with a twofold to threefold increase in the odds of having combined high structural abnormalities, relative to having neither. Thus, both hypertension and arterial stiffness may contribute to the pathogenesis of white matter disease and Aβ deposition in the brain of elderly adults without dementia.
The observed associations between PWV and Aβ deposition align with autopsy studies of amyloid and vascular pathology in hypertensive individuals and extend previous studies of BP and Aβ deposition. Autopsy studies suggest that amyloid burden and other AD pathology may be associated with hypertension22; lessened with hypertensive treatment,22 such as angiotensin receptor blockers23; and co-occur with vascular pathology in the circle of Willis.24 Recent neuroimaging studies using PiB-PET suggest that BP is positively associated with the extent of Aβ deposition in the brain independent of cardiovascular disease risk factors.5,6 While they differ on whether SBP5 or DBP6 is the more important factor, they agree that arterial stiffness is likely the causal factor. We extend these findings to show that arterial stiffness, as measured by PWV, is associated with Aβ deposition in the brain independent of BP and antihypertensive medication use.
Arterial stiffness is more severe in subjects with hypertension, and may explain the relationship found between hypertension and increased AD pathology.1 Elevated BP is a crude surrogate for the underlying mechanisms relating hypertension and brain structure because hypertension is a result of both increased cardiac output and arterial stiffness. Arterial stiffness is a more direct measure of vascular structure and function. It increases with age and represents the individual's susceptibility to BP elevation and the integrated effects of hypertension over time. PWV is considered the gold standard for measuring stiffness of the elastic central arteries.25 Our results show that central and mixed measures of arterial stiffness are associated with severity of both white matter disease and Aβ deposition in the brain.
The associations between arterial stiffness and abnormalities in brain structure differ by type of brain abnormality and vascular bed. baPWV was more strongly associated with Aβ deposition and cfPWV with WMHv. In contrast, faPWV and hfPWV were not associated with any outcomes. The co-occurrence of high Aβ deposition and high WMH was significantly associated with higher cfPWV and baPWV, independent of SBP. Traditionally, cfPWV has been considered the gold standard for measuring stiffness of the elastic central arteries (primarily the aorta).25 Central elastic arteries tend to have different properties and risk factor associations than peripheral (more muscular) arteries, such as the femoral, brachial, or radial artery. baPWV, which includes central and portions of the peripheral arteries (e.g., femoral artery), is also becoming more widely used. baPWV is more strongly correlated with stiffness of the central arteries (i.e., with cfPWV and hfPWV) than with peripheral arterial stiffness measured by faPWV.26 Accordingly, baPWV and cfPWV are correlated similarly with cardiovascular disease risk factors and clinical events.27 We have reported stronger associations of coronary artery calcification28 and weight loss/insulin reductions20 with baPWV than cfPWV. In the present study and others,20 cfPWV had twice the variance of baPWV, which could explain the lack of association between cfPWV and Aβ status; however, it does not explain why cfPWV was more strongly related with WMHv. Measures of central stiffness may simply be associated more strongly with cardiovascular disease risk and cerebrovascular disease, while baPWV may represent another phenomenon linking systemic vascular stiffness with Aβ deposition in the brain.
Higher central arterial stiffness is associated with greater WMH in the brain.8,10,29–34 baPWV is also associated with WMH8,33 and with silent brain infarction.35 Associations between central arterial stiffness and greater total WMH burden are independent of MAP, age, sex, brain volume, and heart rate.30 Central arterial stiffness and WMH reflect cerebral hypoperfusion. Accordingly, higher central measures are associated with lower cerebral perfusion in the hippocampus and the frontal and parietal white matter,32 as well as WMH burden in specific tracts located in “watershed” regions, which are perfused by arterioles with few interconnections available to preserve blood supply in the presence of ischemia.34
A limitation of this study is that PWV and Aβ deposition were not measured at the same time, with PiB-PET preceding PWV measurement between 1 and 2.5 years. However, this gap may not necessarily limit this cross-sectional observation, since little change in markers of arterial stiffness and cerebrovascular disease is expected during the follow-up time.39–43 However, amyloid deposition may have continued to accumulate.44 We recently obtained repeat PiB-PET scans in the same participants (n = 81) within a year (average 124.5 days) of PWV measures and found similar and stronger associations between PWV and Aβ deposition presented here. Similar to the 2009 scans, follow-up PiB status (2011–12) was unrelated to age, sex, BMI, antihypertensive use, or cognition. Being Aβ-positive in 2011–2012 was associated with higher baPWV (p < 0.001) and faPWV (p = 0.011) but not SBP (p = 0.161) after adjustment for age and sex. In models adjusting for age, sex, BMI, and antihypertensive medication use, each SD increase in baPWV and faPWV corresponded to a fourfold increase in the odds of being Aβ-positive at the follow-up PiB-PET. This study provides important insights into the relationship between systemic arterial stiffness and amyloid deposition in the brain. It is possible that increased arterial stiffening has direct impact on penetrating arterioles of the brain, leading to altered structure and function, with subsequent effects on perivascular amyloid clearance from brain via the CSF drainage along the perivascular space.36 This may contribute to a disruption of vascular dynamics and complicate perivascular flow of Aβ,37 thus indirectly causing decreased Aβ clearance leading to plaque formation.38 Further research is needed to determine the temporality of the relationships between arterial stiffness, cerebrovascular disease, and Aβ deposition.
This study provides insight into the associations between arterial stiffness and Aβ deposition in the brain, which may be independent of BP and therefore hypertension. Our study adds to the growing literature that arterial stiffness is associated with subclinical cerebrovascular disease as indicated by WMHv.39,40 Furthermore, higher arterial stiffness is associated with the co-occurrence of high Aβ deposition and high WMHv, which has been suggested to be a “double hit” that may contribute to the development of AD. Further research is needed to determine if the links between central arterial stiffness and WMH are separate from those linking systemic arterial stiffness and amyloid deposition in the brain.
Supplementary Material
GLOSSARY
- Aβ
β-amyloid
- AD
Alzheimer disease
- baPWV
brachial-ankle pulse wave velocity
- BMI
body mass index
- BP
blood pressure
- cfPWV
carotid-femoral pulse wave velocity
- CI
confidence interval
- DBP
diastolic blood pressure
- faPWV
femoral-ankle pulse wave velocity
- GEMS
Ginkgo Evaluation of Memory Study
- hfPWV
heart-femoral pulse wave velocity
- ICC
intraclass correlation coefficients
- MAP
mean arterial pressure
- MCI
mild cognitive impairment
- OR
odds ratio
- PiB
Pittsburgh compound B
- PWV
pulse wave velocity
- SBP
systolic blood pressure
- WMH
white matter hyperintensities
- WMHv
white matter hyperintensities volume
Footnotes
Supplemental data at www.neurology.org
AUTHOR CONTRIBUTIONS
Dr. Hughes: design, data review and interpretation, drafting of manuscript, drafting figures, manuscript revisions, clinical adjudication of cognitive outcomes. Dr. Kuller: study supervision, data collection, data interpretation, critical review of manuscript. Dr. Barinas-Mitchell: data collection, data interpretation, critical review of manuscript. Dr. Snitz: clinical adjudication of cognitive outcomes, critical review of manuscript. Dr. Klunk: data collection, data interpretation, critical review of manuscript. Dr. Mathis: data collection, data interpretation, critical review of manuscript. Dr. Cohen: review of imaging data, critical review of manuscript. Dr. McDade: data interpretation, critical review of manuscript. Dr. DeKosky: study concept and design, funding, data interpretation, critical review of manuscript. Dr. Lopez: study concept and design, clinical adjudication of cognitive outcomes, data interpretation, critical review of manuscript.
STUDY FUNDING
Supported by NIH grants P50 AG005133, R37 AG025516, and P01 AG025204, and a NIA T32 postdoctoral training grant (T32 AG000181, recipient: T.M. Hughes).
DISCLOSURE
T. Hughes, L. Kuller, E. Barinas-Mitchell, R. Mackey, and E. McDade report no disclosures. W. Klunk is a coinventor of PiB, a technology described in this manuscript. As such, he has a financial interest in the license agreement, which GE Healthcare holds with the University of Pittsburgh. He has served as consultant to GE Healthcare, Janssen, Pfizer, Lilly, AstraZeneca, Wyeth, Roche, and Elan. H. Aizenstein serves on the editorial board of the American Journal of Geriatric Psychiatry. He has served as a consultant for the Advanced Research Institute in Geriatric Mental Health for Cornell University. A. Cohen and B. Snitz report no disclosures. C. Mathis is a coinventor of PiB, a technology described in this article. As such, he has a financial interest in the license agreement, which GE Healthcare holds with the University of Pittsburgh. He has served as a consultant for GE Healthcare, Elan/Wyeth, Novartis, Janssen, Genzyme, Pfizer, Bristol Myers Squibb, IBA, and Baxter Bioscience. S. DeKosky reports no disclosures. O. Lopez has served as a consultant for Lilly, Lundbeck, Merz, Lilly, and Baxter. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Sparks DL, Scheff SW, Liu H, Landers TM, Coyne CM, Hunsaker JC., III Increased incidence of neurofibrillary tangles (NFT) in non-demented individuals with hypertension. J Neurol Sci 1995;131:162–169 [DOI] [PubMed] [Google Scholar]
- 2.Yang C, DeVisser A, Martinez JA, et al. Differential impact of diabetes and hypertension in the brain: adverse effects in white matter. Neurobiol Dis 2011;42:446–458 [DOI] [PubMed] [Google Scholar]
- 3.Rabkin SW. Arterial stiffness: detection and consequences in cognitive impairment and dementia of the elderly. J Alzheimers Dis 2012;32: 541–549 [DOI] [PubMed] [Google Scholar]
- 4.Mathis C, Kuller L, Klunk W, et al. In vivo assessment of amyloid-β deposition in non-demented very elderly subjects. Ann Neurol 2013;73: 751–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Langbaum JBS, Chen KW, Launer LJ, et al. Blood pressure is associated with higher brain amyloid burden and lower glucose metabolism in healthy late middle-age persons. Neurobiol Aging 2012;33: 827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Toledo JB, Toledo E, Weiner MW, et al. Cardiovascular risk factors, cortisol, and amyloid-beta deposition in Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement 2012;8:483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Provenzano FA, Muraskin J, Tosto G, et al. White matter hyperintensities and cerebral amyloidosis: necessary and sufficient for clinical expression of Alzheimer disease? JAMA Neurol 2013;70:455–461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saji N, Shimizu H, Kawarai T, Tadano M, Kita Y, Yokono K. Increased brachial-ankle pulse wave velocity is independently associated with white matter hyperintensities. Neuroepidemiology 2011;36:252–257 [DOI] [PubMed] [Google Scholar]
- 9.King KS, Chen KX, Hulsey KM, et al. White matter hyperintensities: use of aortic arch pulse wave velocity to predict volume independent of other cardiovascular risk factors. Radiology 2013;267:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brisset M, Boutouyrie P, Pico F, et al. Large-vessel correlates of cerebral small-vessel disease. Neurology 2013;80:662–669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.DeKosky ST, Fitzpatrick A, Ives DG, et al. The Ginkgo Evaluation of Memory (GEM) study: design and baseline data of a randomized trial of Ginkgo biloba extract in prevention of dementia. Contemp Clin Trials 2006;27:238–253 [DOI] [PubMed] [Google Scholar]
- 12.Aizenstein H, Nebes R, Saxton J, Price J, Mathis C, Tsopelas JA. Frequent amyloid deposition without significant cognitive impairment among the elderly. Arch Neurol 2008;65:1509–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen AD, Mowrey W, Weissfeld LA, et al. Classification of amyloid-positivity in controls: comparison of visual read and quantitative approaches. Neuroimage 2013;71:207–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price J, Klunk W, Lopresti B, et al. Kinetic modeling of amyloid binding in humans using PET imaging and Pittsburgh Compound-B. J Cereb Blood Flow Metab 2005;25:1528–1547 [DOI] [PubMed] [Google Scholar]
- 15.Zhang Y, Brady M, Smith S. Segmentation of brain MR images through a hidden Markov random field model and the expectation maximization algorithm. IEEE Trans Med Imaging 2001;20:45–57 [DOI] [PubMed] [Google Scholar]
- 16.Jenkinson M, Pechaud M, Smith S. BET2: MR-based estimation of brain, skull and scalp surfaces. Eleventh annual meeting of the Organization for Human Brain Mapping; 2005; Toronto
- 17.Wu M, Rosano C, Butters M, et al. A fully automated method for quantifying and localizing white matter hyperintensities on MR images. Psychiatry Res 2006;148:133–142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Snitz BE, Weissfeld LA, Lopez OL, et al. Cognitive trajectories associated with beta-amyloid deposition in the oldest-old without dementia. Neurology 2013;80:1378–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortez-Cooper MY, Supak JA, Tanaka H. A new device for automatic measurements of arterial stiffness and ankle-brachial index. Am J Cardiol 2003;91:1519–1522 [DOI] [PubMed] [Google Scholar]
- 20.Hughes TM, Althouse AD, Niemczyk NA, Hawkins MS, Kuipers AL, Sutton-Tyrrell K. Effects of weight loss and insulin reduction on arterial stiffness in the SAVE trial. Cardiovasc Diabetol 2012;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamashina A, Tomiyama H, Takeda K, et al. Validity, reproducibility, and clinical significance of noninvasive brachial-ankle pulse wave velocity measurement. Hypertens Res 2002;25:359–364 [DOI] [PubMed] [Google Scholar]
- 22.Hoffman LB, Schmeidler J, Lesser GT, et al. Less Alzheimer disease neuropathology in medicated hypertensive than nonhypertensive persons. Neurology 2009;72:1720–1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hajjar I, Brown L, Mack WJ, Chui H. Impact of Angiotensin receptor blockers on Alzheimer disease neuropathology in a large brain autopsy series. Arch Neurol 2012;69:1632–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yarchoan M, Xie SX, Kling MA, et al. Cerebrovascular atherosclerosis correlates with Alzheimer pathology in neurodegenerative dementias. Brain 2012;135:3749–3756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Laurent S, Cockcroft J, Van Bortel L, et al. Expert consensus document on arterial stiffness: methodological issues and clinical applications. Eur Heart J 2006;27:2588–2605 [DOI] [PubMed] [Google Scholar]
- 26.Tsuchikura S, Shoji T, Kimoto E, et al. Brachial-ankle pulse wave velocity as an index of central arterial stiffness. J Atheroscler Thromb 2010;17:658–665 [DOI] [PubMed] [Google Scholar]
- 27.Tanaka H, Munakata M, Kawano Y, et al. Comparison between carotid-femoral and brachial-ankle pulse wave velocity as measures of arterial stiffness. J Hypertens 2009;27:2022–2027 [DOI] [PubMed] [Google Scholar]
- 28.Venkitachalam L, Mackey RH, Sutton-Tyrrell K, et al. Elevated pulse wave velocity increases the odds of coronary calcification in overweight postmenopausal women. Am J Hypertens 2007;20:469–475 [DOI] [PubMed] [Google Scholar]
- 29.Matsumoto M, Inoue K, Moriki A. Associations of brachial-ankle pulse wave velocity and carotid atherosclerotic lesions with silent cerebral lesions. Hypertens Res 2007;30:767–773 [DOI] [PubMed] [Google Scholar]
- 30.Henskens LH, Kroon AA, van Oostenbrugge RJ, et al. Increased aortic pulse wave velocity is associated with silent cerebral small-vessel disease in hypertensive patients. Hypertension 2008;52:1120–1126 [DOI] [PubMed] [Google Scholar]
- 31.Ohmine T, Miwa Y, Yao H, et al. Association between arterial stiffness and cerebral white matter lesions in community-dwelling elderly subjects. Hypertens Res 2008;31:75–81 [DOI] [PubMed] [Google Scholar]
- 32.Tarumi T, Shah F, Tanaka H, Haley AP. Association between central elastic artery stiffness and cerebral perfusion in deep subcortical gray and white matter. Am J Hypertens 2011;24:1108–1113 [DOI] [PubMed] [Google Scholar]
- 33.Hatanaka R, Obara T, Watabe D, et al. Association of arterial stiffness with silent cerebrovascular lesions: the Ohasama study. Cerebrovasc Dis 2011;31:329–337 [DOI] [PubMed] [Google Scholar]
- 34.Rosano C, Watson N, Chang Y, et al. Aortic pulse wave velocity predicts focal white matter hyperintensities in a biracial cohort of older adults. Hypertension 2013;61:160–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saji N, Kimura K, Shimizu H, Kita Y. Association between silent brain infarct and arterial stiffness indicated by brachial-ankle pulse wave velocity. Intern Med 2012;51:1003–1008 [DOI] [PubMed] [Google Scholar]
- 36.Marin-Padilla M, Knopman DS. Developmental aspects of the intracerebral microvasculature and perivascular spaces: insights into brain response to late-life diseases. J Neuropathol Exp Neurol 2011;70:1060–1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid beta. Sci Translational Med 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Armstrong RA. Classic beta-amyloid deposits cluster around large diameter blood vessels rather than capillaries in sporadic Alzheimer's disease. Curr Neurovascular Res 2006;3:289–294 [DOI] [PubMed] [Google Scholar]
- 39.Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol 2008;105:1652–1660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell GF, Vita JA, Larson MG, et al. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation 2005;112:3722–3728 [DOI] [PubMed] [Google Scholar]
- 41.Seidlerova J, Filipovsky J, Dolejsova M. Determinants of aortic stiffening in elderly subjects: results of a nine-year follow-up. Blood Press; 2013;22:173–178 [DOI] [PubMed] [Google Scholar]
- 42.Carmichael O, Schwarz C, Drucker D, et al. Longitudinal changes in white matter disease and cognition in the first year of the Alzheimer disease neuroimaging initiative. Arch Neurol 2010;67:1370–1378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maillard P, Carmichael O, Fletcher E, Reed B, Mungas D, DeCarli C. Coevolution of white matter hyperintensities and cognition in the elderly. Neurology 2012;79:442–448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Villemagne VL, Burnham S, Bourgeat P, et al. Amyloid beta deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer's disease: a prospective cohort study. Lancet Neurol 2013;12:357–367 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


