Abstract
The Viable But Non Culturable (VBNC) state has been thoroughly studied in bacteria. In contrast, it has received much less attention in other microorganisms. However, it has been suggested that various yeast species occurring in wine may enter in VBNC following sulfite stress.In order to provide conclusive evidences for the existence of a VBNC state in yeast, the ability of Saccharomyces cerevisiae to enter into a VBNC state by applying sulfite stress was investigated. Viable populations were monitored by flow cytometry while culturable populations were followed by plating on culture medium. Twenty-four hours after the application of the stress, the comparison between the culturable population and the viable population demonstrated the presence of viable cells that were non culturable. In addition, removal of the stress by increasing the pH of the medium at different time intervals into the VBNC state allowed the VBNC S. cerevisiae cells to “resuscitate”. The similarity between the cell cycle profiles of VBNC cells and cells exiting the VBNC state together with the generation rate of cells exiting VBNC state demonstrated the absence of cellular multiplication during the exit from the VBNC state. This provides evidence of a true VBNC state. To get further insight into the molecular mechanism pertaining to the VBNC state, we studied the involvement of the SSU1 gene, encoding a sulfite pump in S. cerevisiae. The physiological behavior of wild-type S. cerevisiae was compared to those of a recombinant strain overexpressing SSU1 and null Δssu1 mutant. Our results demonstrated that the SSU1 gene is only implicated in the first stages of sulfite resistance but not per se in the VBNC phenotype. Our study clearly demonstrated the existence of an SO2-induced VBNC state in S. cerevisiae and that the stress removal allows the “resuscitation” of VBNC cells during the VBNC state.
Introduction
Microorganisms, like all living organisms, naturally respond to changing environmental conditions. They display a remarkable ability to adapt to certain physical and chemical stresses in their environment. Survival mechanisms are activated following the detection of environmental signals and generate a complex adaptive response that leads to a state of tolerance and thus survival under sub-optimal or even sub-lethal conditions [1]. When the environmental conditions threaten their survival or prevent them from living in optimal conditions, the cells are described as stressed [2]. This notion of stress plays a fundamental role in the survival of microorganisms in foodstuff. Giraffa et al. [3] argued that the ability of microorganisms to grow, survive and display a metabolic activity in foodstuffs is the result of stress response.
However, between the unstressed state and death, different physiological states have been described: viable and culturable, injured, dormant, viable but non culturable (VBNC) and dead [4]. These physiological adaptations require a variable response time depending on the intensity and abruptness of exposure to the stress-inducing factor(s). The VBNC state, which has been extensively studied in bacteria, is characterized by an inability of the cells to grow on culture media, even though they are still viable and maintain a detectable metabolic activity [5]. This state is reversible upon return of favorable conditions. Various environmental factors can induce entry into VBNC state: temperature [6], [7], the physiological age of the culture, salinity [8], the oxygen content [9], light and ventilation [10]. Most studies on VBNC cells have focused on pathogenic bacteria. More than 60 bacterial species are described as being able to enter into a VBNC state, Gram-positive (e.g. Listeria monocytogenes, Enterococcus, Micrococcus luteus) and Gram negative (e.g. Escherichia coli, Vibrio cholerae, Vibrio vulnificus, Legionella pneumophila, Campylobacter jejuni, Salmonella enterica, Pseudomonas aeruginosa, Helicobacter pylorii) [11]. In contrast, the VBNC state has received much less attention in other microorganisms.
The existence of a VBNC state comparable to that described in bacteria has been suggested for the yeast Saccharomyces cerevisiae [12], [13]. A loss of culturability but not of viability has indeed been reported following an electrolytic low amperage shock and suggesting a physiological state comparable to the bacterial VBNC state [14]. Similarly, Bleve et al. [15] detected the presence of S. cerevisiae in a VBNC-like state in pasteurized foodstuffs. In addition, an ecology study conducted during alcoholic fermentation of sweet wines, suggested the presence of cells in a VBNC state in Candida stellata [16]. Sulfur dioxide (SO2) has been identified as the chemical stress factor inducing VBNC state in Brettanomyces bruxellensis grown in a wine synthetic medium [17]–[19]. The same observations were made for S. cerevisiae and Zygosaccharomyces bailii [20]. In order to sustain the hypothesis that the VBNC state is a physiological survival mechanism, it ultimately requires demonstrating the recovery of the culturable state from a VBNC population cells after removal of the stress factor [11], [21]. This resuscitation process is often triggered simply by removal of the stress that initially induced the VBNC response [11], [18]. There has also been numerous reports of resuscitation induced by other mechanisms such as nutrient addition [22], temperature upshift [23] and heat shock [24]. However, most of these resuscitation processes were successful only with cultures which had been in the VBNC state for only a short period of time [17], [21], [25]. In the case of wine yeasts that entered into a VBNC state as a response to SO2 exposure, various authors have shown that a substantial decrease in molecular SO2 concentration induced resuscitation [17], [19], [20].
A number of methods have been employed to examine the viability of non culturable cells in order to suit different needs. The viability of bacteria can indeed be assessed in populations (bulk assay) or in single cells (cytological assay) [4], [25]. The latter appear to be preferred since it is based on growth-independent viability techniques such as the assessment of cell viability by the maintenance of stable cellular structure. These methods include the use of nucleic acid stains, redox indicators, membrane potential probes or metabolic indicators such as fluorescein diacetate that can be detected by fluorescence microscopy [19], [20] or flow cytometry [17].
In this study, we evaluated the effect of SO2 on the entry of S. cerevisiae cells into the VBNC state, the resuscitation capability of VBNC S. cerevisiae cells using a flow cytometry and trying to demonstrate that the recovery of culturability is due to a true resuscitation and not to the presence and growth of a few residual cells with a normal metabolism. Finally, we investigated the role of SSU1 in the VBNC state.
Materials and Methods
Strains, Plasmids and Culture Conditions
The different bacteria, yeast strains and plasmid used in this study are listed in Table 1.
Table 1. Yeast strains used in this study.
| Strains and plasmid | Genotype/Description | Reference |
| S. cerevisiae S288C | MATα SUC2 gal2 mal mel flo1 flo8-1 Δ hap1 | |
| S. cerevisiae BYD4742 | S288C derivative, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 | [46] |
| S. cerevisiae BYD4742 Δssu1 | BYD4742 derivative, MATα his3Δ1 leu2Δ0 lys2Δ0 ura3Δ0 ssu1::KanMX4 | EUROSCARF deletion library* |
| Escherichia coli DH5α | [F−φ80lacZΔM15Δ(lacZYAargF) U169 deoR recA1 endA1 hsdR17(rk−, mk+) phoA supE44 thi-1gyrA96 relA1 λ] | GIBCO-Invitrogen Life technologies, Mowbray,South Africa |
| pCEL13 | 2 µm ApR URA3 PGK1P–PGK1T | [27] |
| pCEL13-SSU1 | 2 µm ApR URA3 PGK1P-SSU1-PGK1T | This study |
Plasmids were constructed and amplified in Escherichia coli DH5α, grown in Luria Bertani (LB) medium (Biolab diagnostics, Wadenville, South Africa). The medium was supplemented with 100 mg/L ampicillin for the selection of resistant bacteria when appropriate. S. cerevisiae (S288C, BYD4742 and BYD4742Δssu1) strains were grown in Yeast Peptone Dextrose (YPD) (10 g/L yeast extract, 10 g/L Bacto-peptone, 20 g/L glucose) at 28°C. For the selection of yeast transformants, Synthetic Complete (SC) medium containing 20 g/L glucose, 6.7 g/L yeast nitrogen base with ammonium sulfate and amino acids (Difco Scientific group, Waterfall Park, South Africa) supplemented with 60 µg/mL leucine and 30 µg/mL lysine to apply a uracil auxotroph.
DNA Preparation and Analysis
Chromosomal DNA from S. cerevisiae BYD4742 strain was isolated from overnight culture grown in YPD at 30°C [26]. The SSU1 gene was amplified by polymerase chain reaction (PCR) using the 5′ScSSU1fw (GGATCCATGGTTGCCAATTGGGTACTT) and 3′ScSSU1rev (CTCGAGTTATGCTAAACGCGTAAAATCTAGAG) primers in an Applied Biosystems 2720 thermal cycler. Phusion DNA polymerase enzyme (Finnzymes, Finland) and Phusion buffer (Finnzymes, Thermo Scientific, Pretoria, South Africa) with MgCl2 were used. The reaction mixture contained Phusion DNA polymerase enzyme (1 U), Phusion buffer (1X), 250 µM of each nucleotide (dNTP), 200 ng genomic DNA, 0.25 µM of each primer, and 0.2 mM MgCl2. The PCR program is consisted of a 30 s initial denaturation cycle at 98°C (initial denaturation), followed by 35 cycles of 98°C for 10 s (denaturation), 58°C for 45 s (annealing), 72°C for 50 s (elongation). The program ended with a final 10 min extension at 72°C (final elongation).
The amplicons obtained were cloned into a pJET1.2 using the CloneJet PCR Cloning Kit (Thermo Scientific) according to the manufacturer’s instructions. Plasmid DNA was isolated from positive transformants of E. coli DH5α using the Qiaprep Spin Miniprep Kit (Qiagen, Whitehead Scientific, Cape Town, South Africa). Both strands were sequenced in an ABI 3130XL Genetic Analyzer at the Central Analytical Facility (Stellenbosch University) using the pJET1.2 Forward and Reverse sequencing primers.
Constructing Overexpression Vectors
The SSU1 gene was then subcloned into the pCEL13 yeast expression vector [27] (Table 1) as follows: SSU1 was excised from pJET1.2 restriction with BamHI and XhoI (Roche Diagnostics, Randburg, South Africa) and ligated into the BglII and XhoI sites of the pCEL13 expression vector respectively to yield a plasmid named pCEL13-SSU1. Restriction endonuclease-digested DNA was eluted from agarose gels by using the ZymocleanTM gel recovery kit (Zymo research, USA) according to the manufacturer’s instructions. Standard methods were used for the restriction and ligation of DNA, plasmid transformation into E. coli, and agarose-gel electrophoresis [28].
Yeast Transformation
S. cerevisiae, strain BYD4742Δssu1 was transformed with pCEL13-SSU1. Yeast transformation was conducted using an electroporation method as previously described [29]. The plasmids were maintained as autonomously replicating plasmids in the yeast cells by growing yeast cells cultured in uracil deficient media. Transformation was verified by colony PCR analysis using the 5′KPNPGK-631(GGGGTACCCTTTATTTTGGCTTCACCC) and 3′PGKKPN-1378 (CGCGGGGGTACCGATAAATAATAGTCTATATATACG) primers. The reaction was performed in 50 µl using 1×Taq buffer (Promega Corp., USA), 250 µM of each nucleotide dNTPs, 1.5 mM MgCl2, and 1 unit of Taq DNA polymerase (Promega Corp., USA) with the following cycling conditions: 10 min initial denaturation cycle at 98°C (initial denaturation), followed by 30 cycles of 98°C for 10 s (denaturation), 58°C for 30 s (annealing), 72°C for 50 s (elongation). The program ended with a final 10 min extension at 72°C (final elongation). PCR products were resolved on 1% agarose gel prepared with 1×TBE buffer and 1 µM of ethidium bromide and visualized under UV-light; the relative molecular length of the PCR product was estimate to be about 2 Kpb in order to validate the yeast transformation (results not shown).
Adaptation of Different Strains to the Synthetic Wine Medium
S. cerevisiae S228C, BYD4742 and BYD4742Δssu1strains were grown on YPD, S. cerevisiae BYD4742Δssu1pCEL13-SSU1 was grown on SC at 28°C, for 5 days as starter inocula.
For S. cerevisiae S228C,VBNC studies were performed in synthetic wine (SW) (8% ethanol, 3 g/L D-L malic acid, 0.01% acetic acid, 0.1 g/L potassium sulfate, 0.025 g/L magnesium sulfate, 1 g/L yeast extract, 1.5 g/L glucose, 1.5 g/L fructose). VBNC studies for S. cerevisiae BYD4742, BYD4742Δssu1 and BYD4742Δssu1 pCEL13-SSU1 was performed in modified synthetic wine (MSW) (8% ethanol, 3 g/L D-L malic acid, 0.01% acetic acid, 0.1 g/L potassium sulfate, 0.025 g/L magnesium sulfate, 1.5 g/L glucose, 1.5 g/L fructose, 6.7 g/L yeast nitrogen base with ammonium sulfate and amino acids supplemented with 60 µg/mL leucine and 30 µg/mL lysine) supplemented with 50 µg/mL uracil for the culture of S. cerevisiae BYD4742, BYD4742Δssu1 strains.
The pH was adjusted to 3.5, using 2 M NaOH and the medium was filter-sterilized using 0.2 µm filters (Millipore, Molsheim, France). One single colony was inoculated into 10 mL of SW-YPD (50∶50) (S. cerevisiae S228C) or in 10 mL of MSW-SC (50∶50) (S. cerevisiae BYD4742, BYD4742Δssu1, and BYD4742Δssu1 pCEL13 SSU1) and incubated at 28°C for 3 days. 5.105 cell/mL from this preculture were inoculated into 1 L SW or MSW, depending on the strain, and incubated at 28°C for 3 days in order to obtain approximately 107 cell/mL.
Culturability and Viability Assays
Samples of S. cerevisiae suspensions were taken at various time points during incubation at 28°C in SW or MSW, for the determination of total, viable and culturable populations. Cell culturability was assessed by a spread plating procedure on YPD agar or SC agar depending on the strain. The percentage of cells that were viable was expressed as total cell counts determined by flow cytometry (FCM). Two fluorescent dyes, namely fluorescein diacetate (FDA) and FUN-1, were used to evaluate the viability of S. cerevisiae using FCM. FDA is a lipophilic, uncharged and non-fluorescent substrate for cellular esterase that cleaves FDA inside living cells to release green fluorescent fluorescein (emission at 520 nm). FDA is therefore used to monitor cellular esterase activity and to determine the viability of cell populations. For the staining procedure, 0.5 mL of cultured cells was added to 0.5 mL of FDA buffer ((0.5 M Na2HPO4 (Sigma 255793, France; pH 7.4) and 0.5 M NaH2PO4(Sigma S2554, France); pH 7)) to which 1.5 µL of FDA at 10 µM in acetone (Sigma F737, USA) was added in order to reach a final concentration of 15 µM, and the cells were then incubated for 15 min at room temperature in the dark before being analyzed by FCM.
Furthermore, another viability probe (FUN-1) (Invitrogen F-7030) was used in order to validate the presence of the metabolic activity in the non culturable cells. FUN-1 [2 chloro 4 (2,3 dihydro 3 methyl (benzo 1,3 thiazol-2-yl) methylidene) 1 phenylquinolinium iodide] is a fluorescent probe that belongs to a class of halogenated asymmetric cyanine dyes and is essentially non-fluorescent in aqueous solution. FUN-1 stains nucleic acids, producing a green to green-yellow fluorescence in membrane compromised dead yeast cells [30]. In metabolically active yeast, cylindrical intravacuolar structures (CIVS) are produced after less than 1 h exposure to FUN-1 [30]. This stain gives rise to the formation of CIVS structures in the vacuoles of metabolically active yeast cells grown and stained under either oxidative or fermentative conditions [30]. These structures often appear to move within a vacuolar space and are red when excited at 470–590 nm. To stain cells with FUN-1 different suspensions of live, dead, and non culturable yeast cells were analyzed. Saccharomyces cerevisiae S288C cells were washed in sterile PBS (130 mM NaCl (Sigma-Aldrich #S9888, St Quentin Fallavier, France) 5 mM NaH2PO4 (Sigma-Aldrich #S2554) and 5 mM Na2HPO4 (Sigma-Aldrich #255793) pH 7.2) and a portion was killed using Natamycin (yeast cells treated with Natamycin (Delvocid) (Humeau, France), for 60 min at 28°C). The absence of viability was confirmed by absence of growth on YPD agar media and by FCM analysis using FUN-1. Live, dead and non culturable yeasts were stained separately using FUN-1, 1 mL of each S. cerevisiae suspension was washed twice with PG solution (PBS pH 7.2 containing 2% glucose). A centrifugation at 10000 g for 5 min was performed and the pellet was resuspended in PG solution (this solution ensures that yeasts remain metabolically active during the experiment). The cells were then incubated with FUN-1 at a final concentration of 15 µM for 30 min at 28°C. Cells were analyzed by FCM. A dot plot of Red fluorescence (y-axis) over Green fluorescence (x-axis) was prepared.
Flow Cytometry Analysis
FCM samples were analyzed using a Guava EasyCyte Plus SSC4C flow cytometer (Guava Technologies, Hayward). This instrument is equipped with a 488-nm, 25-mW laser line, forward scatter (FSC, for cell size) and side scatter (SSC, for granularity) detectors; green fluorescence was collected on the FL 1 channel using a 525-nm (±30 nm) band-pass filter red fluorescence was collected on the FL 3 channel using a 680-nm (±30 nm) band-pass filter. This instrument allows determining accurate cell numbers and population percentages, without the need for reference beads, as described by the manufacturer using only the Guava Cytosoft data acquisition and analysis software. For all analyses, a minimum of 5,000 events was acquired, and all samples were collected as logarithmic signal. Experiments were performed in duplicate and included an unlabeled sample as a control in 96-well plates. Data were analyzed using the Guava Cytosoft data acquisition and analysis software version 5.0 and FlowJo software version 7.6.
Induction of Entry into and Exit from the VBNC State
Based on studies that have been carried out previously [17], [20], SO2 was used to induce the VBNC state. In wine, different species of SO2 are in a pH-dependent equilibrium: HSO3 −, SO3 2− and molecular SO2. The latter is the main antimicrobial species of SO2 [31]. When pH decreases, the concentration of molecular SO2 increases as does the antimicrobial strength for a given total SO2 concentration [31].
Entry into the VBNC state was induced by adding different concentrations of molecular SO2 (ranging between 0.1 mg/L and 4.5 mg/L). Desired molecular SO2 concentration was obtained using potassium metabisulfite solution. The level of potassium metabisulfite to be added was determined as reported previously [32] taking into account, the pH of the medium and the pKa of SO2. Exit from the VBNC state was induced at different time intervals after addition of SO2 (i.e. 3, 7, 14, 21, and 30 days) by adjusting the pH to 4.0 via the addition of 2 M NaOH. A pH of 4.0 was indeed found sufficient to bring the concentration of molecular SO2 close to 0. All of our studies of entry into and exit from the VBNC state were performed in triplicate. The percentage of viable cells was calculated as follows: % viability = (viable cell count/total cell count)×100.
FDA Reliability Assay
A synthetic wine was inoculated with S. cerevisiae S288C strain to a final concentration of 5.105 CFU/mL and incubated for 3 days at 28°C to obtain approximately 107 CFU/mL. Thereafter, different lethal stresses such as Natamycin (25 mg/L) and SO2 (10 g/L) were applied. Every 5 min 1 mL of cells was centrifuged (13,000 g for 5 min at 25°C), the pellet was rinsed twice in PBS and cell culturability was assessed by a spread plating procedure on YPD media. Green fluorescent intensity was determined by flow cytometry using FDA.
VBNC Cell Cycle Analysis
A comparison of the cell cycle profiles of cells in VBNC state and cells exiting the VBNC state was carried out in order to show the absence of cell proliferation during the exit from the VBNC state using FCM and propidium iodide (PI), a red fluorescent probe (635 nm emission) that binds to the nucleic acid [33], [34]. 1 mL of cell suspension of S. cerevisiae S288C from exponentially growing culture in SW (control), VBNC and culturable cells exiting from the VBNC state were centrifuged for 5 min at 10,000 g, the pellet was suspended in 1 mL cold 70% ethanol and the tubes were stored for 3 hours at 4°C. Cells were suspended in 1 mL 50 mM citrate buffer pH 7 (Sigma-Aldrich #S4641) after 5 min centrifugation at 10,000 g. A second centrifugation for 5 minutes at 10,000 g was performed and the cells were suspended in 1 mL 50 mM citrate buffer pH 7 containing 0.25 mg/mL RNase A (Sigma-Aldrich #R4875), to ensure DNA-specific binding as PI can stain both double-stranded RNA and DNA [35]. Incubation for 1 h at 50°C was then carried out. In order to stain cells with PI, the tubes were centrifuged again for 5 min at 10,000 g and the pellet was resuspended in citrate buffer pH 7 containing 8 µg/mL PI (Sigma-Aldrich #81845) and stored at 4°C for 3 days. All analyses were carried out in triplicate at a concentration of 106 cell/mL. PI is detected in the 575/26 nm channels on the BD LSRII which is equipped with a 488-nm, 22-mW laser line, forward scatter (FSC) and side scatter (SSC) detectors. Initial cell population gating is placed on FSC vs SSC (cell size vs granularity). This cell population gate was then placed on PE 575/26 nm-W(width) vs PE 575/26 nm-A(Area) plot. Doublets appear to the right of single cell analysis (gate P2). Single cell gate P2 was then displayed as a histogram using PE 575/26 nm-A parameter. For all analyses, a minimum of 10,000 events was acquired, and all samples were collected as linear signal. Cell Cycle analysis of research samples was adequately done using the FlowJo software.
VBNC Exit Rate Assay
A comparison of the cell generation time and exit rate of the VBNC cells was carried out in order to further verify the absence of cell proliferation during the exit from the VBNC state. A filtered SW pH 4.0 obtained from a culture of S. cerevisiae S288C (14 days, in synthetic wine containing 8% ethanol) was inoculated with the same strain to a final concentration of 104 CFU/mL. This culture was used to determine the generation time (doubling time of the biomass in the exponential phase) of S. cerevisiae S288C, under the same experimental conditions, during the exit from the VBNC state. To determine the generation time (G), the optical density at 600 nm as well as plate counting on YPD agar were determined every 2 h and compared to the exit rate of the VBNC state which was determined after the removal of the SO2 stress as described above. The generation time and the exit rate were calculated according to the following formula: G = ln(2)/μ (max) wih ln(N2)-ln(N1) = μ (max) (t2-t1), (N2 is cell number at t2 and N1 is the cell number at t1).
Results and Discussion
Evidence for a VBNC State in S. cerevisiae (Induction and Exit)
SO2 was used as a stress factor in an attempt to induce the VBNC state in S. cerevisiae. FCM counts of total or viable cells using FDA and culturable cell counts were compared in order to monitor the entry of S. cerevisiae S288C cells into the VBNC state. In the absence of SO2, more than 95% of total cells remained viable and cultivable during the first three days (Fig. 1A). Entry into the VBNC state was assayed by incubation of the cells with different concentrations of molecular SO2, ranging from 0.1 to 4.5 mg/L. When 4.5 mg/L of molecular SO2 were added 3 days after synthetic wine was inoculated (Time 0), the viability and the culturability of cells decreased rapidly and all viable cells became non culturable after 48 h (Fig. 1B). When applying lower concentrations of SO2, some viable cells always remained culturable (data not shown). In the first 3 days following the addition of SO2, a decrease of viability from 4.2×106 to 2.2×106 cells/mL was observed and could be explained by the fact that some cells are more sensitive to SO2 than others. In the third day (72 h) following the sulfite stress, no more colonies were detected on YPD medium. The difference between the percentage of culturable cells and viable cells suggests that a significant proportion of cells were in a VBNC state (Fig. 1B). For strain S288C, 52%±20% (2.2×106 cells/mL)in average of the total population was in VBNC state after 3 days and 1%±0,5% (4.2×104 cells/mL) remained in a VBNC state 36 days after stress exposure while the rest of the population died.
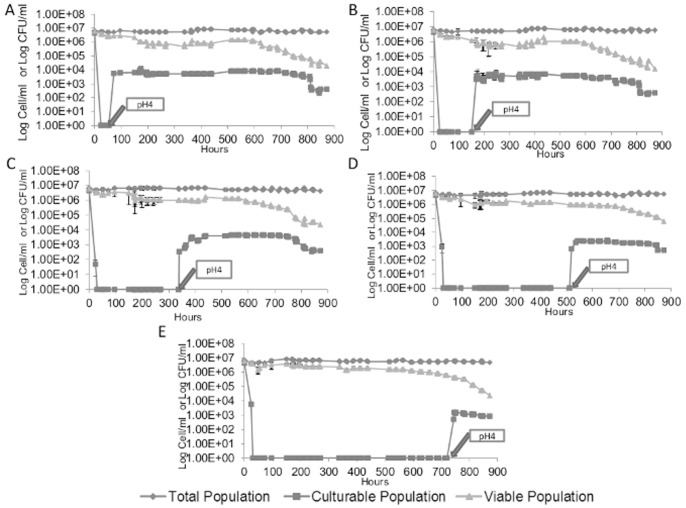
Figure 1. Changes in the total cell population (♦) culturable population (▪), and viable population (▴) of a culture of S. cerevisiae S288C on incubation at 28°C.
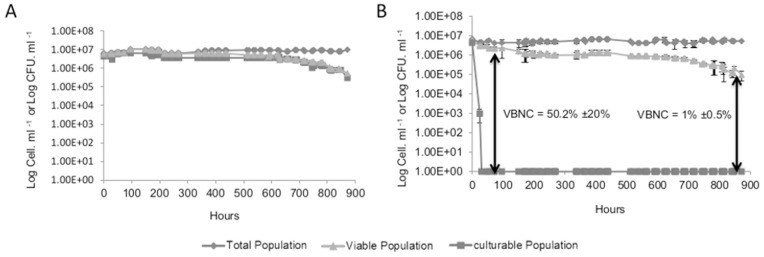
Panel A shows the growth control condition in synthetic wine. Panel B shows the induction of VBNC state in S. cerevisiae S288C in synthetic wine with the addition of 4.5 mg/L molecular SO2 at time 0. Value 1 corresponds to an undetectable number (less to 10 CFU/mL).The values presented are the average of three replicates of three separate experiments.
The ability of cells to exit from the VBNC state was investigated at different days (3, 7, 14, 21 and 29 days or 72 h, 168 h, 336 h, 504 h, 696 h respectively) (Fig. 2) by increasing the pH from 3.5 to 4.0 in order to decrease the molecular SO2 concentration [17]. In order to rule out the effect of pH on VBNC state, the effect of rising the pH on yeast growth dynamic has been checked (Fig. S1). It appears that pH increase did not lead to VBNC cells. One day after the pH-induced drop of the molecular SO2 concentration, approximately 1%±0,5% of the cells that initially entered into a VBNC state recovered culturability, regardless of the period of time they have been in the VBNC state (72 h, 168 h, 336 h, 504 h, 696 h ) (Fig. 2). The percentage of yeast recovering culturability started to decrease after few days depending on the time they were kept in VBNC state. It can be speculated that, all cells did not resuscitate because of the heterogeneity of physiological states within the yeast population [36].
Figure 2. Resuscitation of S. cerevisiae S288C from the VBNC state.
Total cell counts (♦), culturable counts (▪), and viable counts (▴) are shown. Resuscitation was induced by removal of the molecular SO2 at different time intervals after entry into VBNC state (i.e. A: 3 days, B: 7 days, C: 14 days; D: 21 days; E: 30 days). Value 1 corresponds to an undetectable number (less to 10 CFU/mL). The values presented are the average of three replicates of three separate experiments.
In the current study, we managed to confirm the ability of S. cerevisiae to survive in a VBNC state over a long period of time (36 days, 864 h). The results indicate that S. cerevisiae becomes non culturable after three days in response to SO2 exposure but 52%±20% of the initial population remains viable as assessed by FDA probe (Fig. 1B). This observation agrees with the antimicrobial activity of SO2 [37] and with the hypothesis according to which SO2 induces a viable but non culturable state in S. cerevisiae [20]. Moreover, stress removal by increasing the pH of the growth medium allows VBNC cells to resuscitate (i.e. recover culturability) (Fig. 2).
Metabolic Activity in Non-culturable Cells
In order to ensure that the green fluorescence intensity observed in VBNC cells is a good reflection of metabolic activity and not a residual esterase activity, the green fluorescence intensity was measured in dead cells obtained using 2 lethal chemicals such as the exposure to natamycin (25 mg/L) or SO2 (10 g/L). Our results show that after 150 min of treatment with natamycin (25 mg/L) (Fig. 3) or 45 min of treatment with SO2 (10 g/L) (Fig. S2) no cells presented a green fluorescence. This indicates that the green fluorescence reflects a true metabolic activity and not a residual esterase activity. This validates that cells that are considered in VBNC state after being exposed to SO2 stress and still detectable by FCM (for a longer period of time more than 24 h after the loss of their culturability) are metabolically active in a VBNC state (Fig. 2).
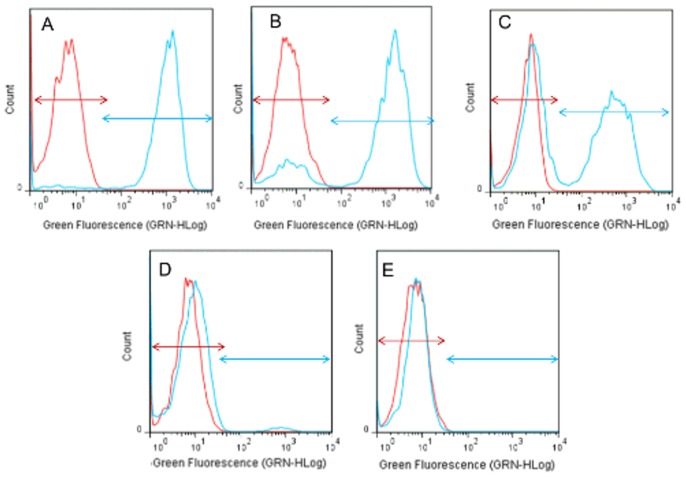
Figure 3. FCM histograms of S. cerevisiae S288C cells stained with FDA.
The cells were incubated with 25/L natamycin in Synthetic wine at 28°C. After 30 (B); 60(C); 120(D) and 150(E) min, the cells were collected, and the cell Green fluorescence intensity was analyzed by FCM, Panel A represents control cells in the absence of SO2 (0 min). The Green fluorescence intensity (GRN-HLog) is represented on the x-axis, and cell counts are represented on the y-axis. Panels show the fluorescence of S. cerevisiae S288C before (red arrow; self-fluorescence) and after (blue arrow) staining with FDA. One representative experiment of the three performed is shown.
The analysis by FCM using FUN-1 of viable, dead and non cultrable (Viable and culturable cells treated with 4.5 mg/mL of SO2) cells of S. cerevisiae S288C was performed. The Green and the Red-labeled populations were spatially resolved in dot plots of FL1 and FL3. Analysis by FCM of the dead cells (treated with natamycin) stained by FUN-1 shows that more than 97.87% of cells diffused a green to green-yellow fluorescence indicating that the membrane was compromised as provided in a dead yeast cells (Fig. 4A). Analysis of viable (obtained 3 days after sulphite stress in synthetic wine) and culturable cells by FCM after staining by FUN-1 reveals the presence of a red fluoresence in 95.2% of the total population which indicates the formation of CIVS structures in the vacuoles of the metabolically active yeast cells (Fig. 4B).
Figure 4. FCM analysis of S. cerevisiae S288C cells stained with FUN-1.
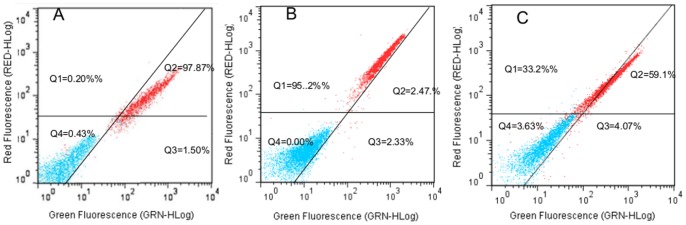
Green fluorescence intensity is shown on the x-axes and red fluorescence intensity is shown on the y-axes. Dot plot (A) shows the fluorescence of dead cells after staining with FUN-1. Dot plot (B) shows the fluorescence of viable and culturable cells after staining with FUN-1. Dot plot (C) shows the fluorescence of VBNC cells after staining with FUN-1. Red-negative cells are contained in quadrants 3 and 4; Red-positive cells are contained in quadrants 1 and 2. Green- positive cells are contained in quadrants 2 and 3; Green-negative cells are contained in quadrants 1 and 4. Red fluorescence was measured at 630 nm (emission) and Green at 525 nm (emission). One representative experiment of the three performed is shown.
33.2%±6% of the non culturable cells analyzed by FCM using FUN-1 displayed a red fluorescence (Fig. 4C). The presence of a red fluorecence in non culturable cells (33.2%±6%) showed that these cells present a CIVS structure that allows us to validate that a significant population within the non culturable cells present a metabolic activity.
VBNC State Validation
Proving the existence of the VBNC phenomenon as a physiological survival mechanism ultimately requires demonstrating the possible recovery of the culturable state from a non culturable population [11], [21]. Indeed, VBNC state can only be a significant means of survival if the cells surviving in this state are able to again recover their ability to multiply. In order to show that the recovery of culturability observed after the removal of the molecular SO2 stress (Fig. 2), is a true resuscitation and not a growth of a few residual viable and culturable cells with normal metabolism, a comparison of the profile of cell cycle in VBNC state just before and immediately after pH adjustment was performed using FCM. In addition, in order to determine the relative cellular DNA content, FCM was used to identify the cell distribution among the various phases of the cell cycle. The analysis of an exponentially growing population of S. cerevisiae S288C in synthetic wine medium using FCM with the DNA binding dye propidium iodide allowed the identification of the different phases of the cell cycle based on the theoretical distribution histogram of cells according to the linear relation between fluorescence intensity and by extrapolation the DNA content (Fig. 5A). 26.1% of the cells were detected with a 2C DNA content corresponding to the G1 phase (fluorescence intensity ≈ 45.103). 43.33% of the cells were detected with a 4C DNA content corresponding to the G2 and M phases (fluorescence intensity ≈ 90.103). Finally, 30.57% of the cells were found in the S phase, synthesizing DNA continuously and displaying a DNA content between 2C and 4C. The analysis of the cell cycle profile of cells in VBNC state (Fig. 5B) and cells exiting the VBNC state (3 days after the removal of the stress, VBNC percentage equal to 68%) (Fig. 5C), showed that most cells are in the S phase (43.3%) for both physiological states and exhibited similar profiles with an absence of a cell proliferation during resuscitation (Fig. 5A and B). Since the cell cycle profile is the same before and after exit from VBNC, this means that no cell multiplication occurred in the synthetic wine. This result together with the fact that after pH rising cells are culturable again (Fig. 2) demonstrated that these cells are able to again recover their ability to multiply.
Figure 5. FCM analysis and cell cycle distribution of S. cerevisiae S288C.
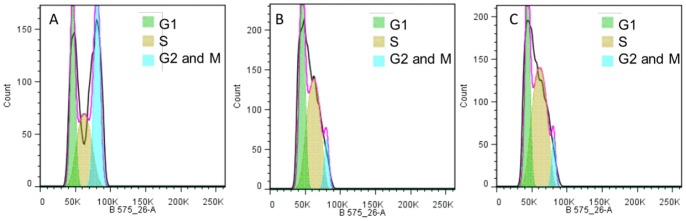
Analysis of S. cerevisiae S288C cell cycle during exponential phase in synthetic wine (Panel A), before (Panel B) and after exiting (Panel C) the VBNC state analyzed by FCM. The profiles Showed dual-variable plots of cell number versus PI uptake. G1 (green), S (brown), and G2/M (blue) cell populations were quantified. The experiment was repeated at least three times and representative data from single experiment is presented.
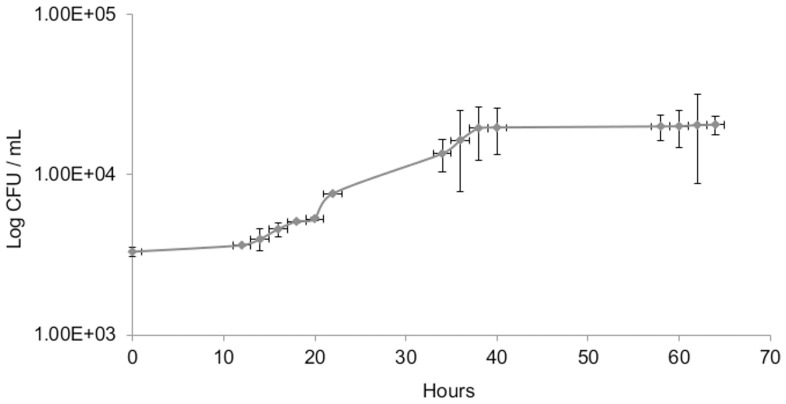
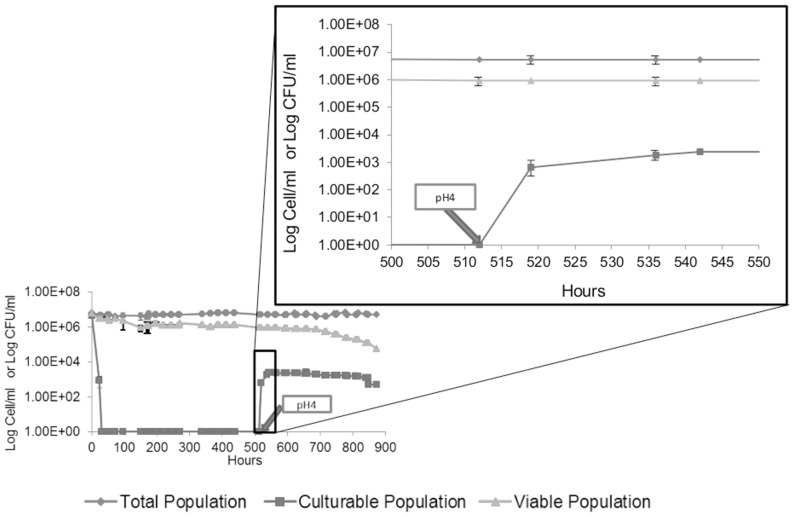
The generation time of S. cerevisiae S288C was determined by inoculating the S288C strain under the same experimental conditions during the exit from the VBNC state and was found to be approximately 10 h (Fig. 6). As, the culturability assay used in our study (100 µL on YPD agar) had a detection limit equivalent to 10 CFU/mL, consequently, during the resuscitation process, at least 56.9 h would have been required to reach a concentration of 5.19×102 CFU/mL after the increasing of the pH, if the observed increase in culturability had been due to the presence of culturable cells. Yet our results show that 5.19×102 CFU/mL of culturable cells were observed only 7 h after the pH increase (Fig. 7). According to the generation time calculated above, no viable and culturable cells would be able to grow up to 5.19×102 CFU/mL in such a short period of time (7 h) (Fig. 7).
Figure 6. Growth of S. cerevisiae S288C in synthetic wine at 28°C.
Error bars indicate the standard deviations of three independent experiments. The generation time is equal to 10 hours.
Figure 7. Exit rate of S. cerevisiae S288C from the VBNC.
Total cell counts (♦), culturable counts (▪), and viable counts (▴) are shown. Resuscitation was induced by removal of the molecular SO2 at 21 days after entry into VBNC state. Value 1 corresponds to an undetectable number (less to 10 CFU/mL). The values presented are the average of three replicates of three separate experiments.
These results therefore validate the hypothesis of the VBNC state which is based on the fact that cells are able to regain their ability to multiply. This resuscitation has been strongly debated [21], [38], [39], as some authors suggest that the recovery of culturability is due to the presence and sudden growth of a few residual cells with a normal metabolism in a population predominantly non culturable. However, the recovery of cell division in a population of VBNC cells was described unambiguously for several bacteria [40], [41]. Cell resuscitation has been clearly demonstrated in vitro, in vivo and in situ [11]. In this study, the removal of environmental stress was sufficient to induce the exit from the VBNC state and the recovery in culturability observed was evidenced as a true resuscitation and not a simple growth of a few residual cells with a normal metabolism.
Role of the Ssu1p Pump in the VBNC State
SO2 resistance mechanisms have been extensively studied in S. cerevisiae. SO2 detoxification, involving the plasma membrane protein Ssu1p, is one of the most efficient resistance mechanisms in this species [42]. Yeasts also tolerate SO2 by means of other systems, such as acetaldehyde production and the up-regulation of sulfite reduction systems [43]. The sulfite pump required for efficient sulfite efflux is encoded by the SSU1 gene. Generally, mutations in SSU1 cause sensitivity, whereas overexpression confers enhanced resistance to sulfite toxicity [44], [45].
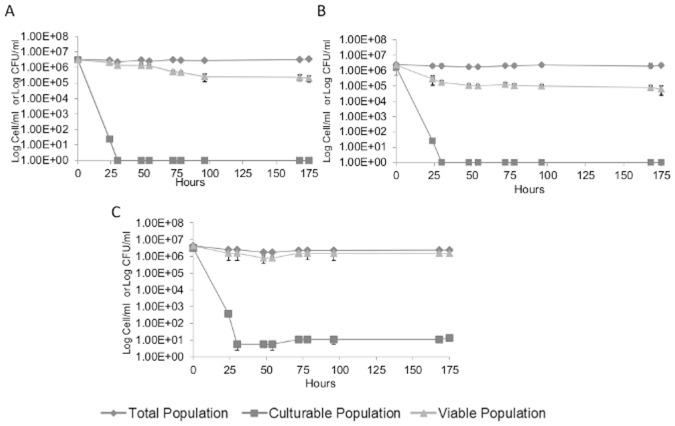
In order to investigate the potential role of Ssu1p in the VBNC state of S. cerevisiae, the VBNC profiles of three strains, namely BYD4742, BYD4742Δssu1 and BYD4742Δssu1 pCEL13-SSU1, were compared. The study was carried out in a modified synthetic wine medium containing 4.5 mg/L of molecular SO2. Total and viable cell counts determined by flow cytometry and CFU counts (on SC agar) were compared in order to monitor the difference in the VBNC profile of the three strains. In the absence of SO2, more than 95% of total cells remain viable and cultivable during the first three days (data not shown). Entry into the VBNC state was induced by the addition of SO2 (4.5 mg/L molecular SO2) 3 days after synthetic wine inoculation (Time 0). The total population remained stable over time for all strains and culturability decreases quickly 30 h after SO2 addition to undetectable levels only for BYD4742 and BYD4742Δssu1 (Fig. 8 A and B). However, for BYD4742Δssu1 pCEL13-SSU1, some cells were still found culturable (5.5 cell/mL) even after 30 h of treatment (Fig. 8 C).
Figure 8. The induction of the VBNC state in S. cerevisiae BDY4742 (Panel A), BDY4742Δssu1 (Panel B) and BDY4742Δssu1 pCEL13 SSU1 (Panel C) strain in synthetic wine with the addition of 4.5 mg.L−1 molecular SO2.
Total cell counts (♦), culturable counts (▪), and viable counts (▴).Value 1 corresponds to an undetectable number (less to 10 CFU/mL). The values presented are the average of three replicates of three separate experiments.
Moreover, the cell viability of BYD4742Δssu1 decreased rapidly after the addition of SO2, to less than 10% 30 h after the treatment, whereas the viability of BYD4742 and BYD4742Δssu1 pCEL13-SSU1 strains decreased more slowly in the first few hours following the treatment (i.e. 60% of viability 30 h after treatment). This difference in the response to SO2 exposure between the wild-type, BYD4742Δssu1 pCEL13-SSU1 and BYD4742Δssu1 allows to validate the role of SSU1 in sulfite resistance mechanisms, as previously reported [42] and can be explained by the fact that the SSU1 null mutant accumulated significantly more sulfite than the other strains (wild-type and BYD4742Δssu1 pCEL13-SSU1), which make SSU1 null mutant strain more sensitive to SO2.
However, after 78 h, viability was no more significantly different and identical viability percentages (8%) were observed between the wild-type and BYD4742Δssu1 (Fig. 8 A and B, Fig. 9) whereas 61% of BYD4742Δssu1 pCEL13-SSU1 cells remained viable. This could be explained by the fact that upon sudden exposure to a very high concentration of SO2 such as that required for entry into the VBNC state, the wild-type strain, unlike BYD4742Δssu1 pCEL13-SSU1, does not have enough sulfite pumps in its membrane to efflux enough SO2 and detoxify the intracellular matrix. The overexpression of SSU1 in a SSU1 null mutant using the pCEL13 vector conferred enhanced resistance to sulfite toxicity as previously described [44], [45] ruling out the need for entry into a VBNC state (i.e. more than 99% of viable cells are non culturable). This result allows us to conclude that the SSU1 gene is involved in sulfite resistance but not in the VBNC phenotype of Saccharomyces cerevisiae.
Figure 9. Viability percentage of S. cerevisiae BYD4742 (♦), BYD4742Δssu1 (▪) and BYD4742Δssu1 pCEL13 SSU1(▴) strain during the induction of the VBNC state.
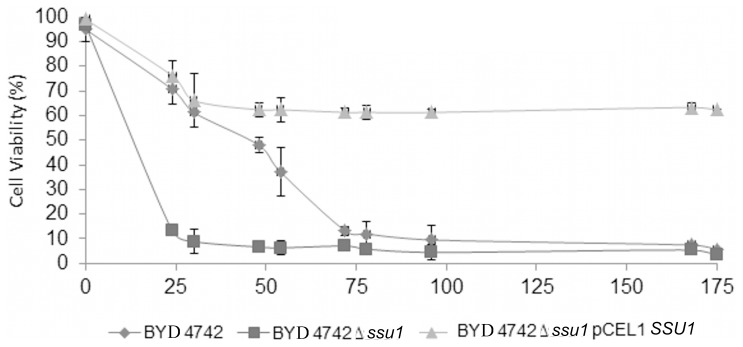
Viability percentage is determined by FCM using FDA. Error bars indicate the standard deviation of three independent experiments.
Conclusion
S. cerevisiae S288C strain was used to generate conclusive evidence for the existence of a VBNC state in yeast, using a sulfite stress (4.5 mg/L molecular SO2). For this purpose, cell count results obtained by FCM were compared to those obtained by plating on culture medium.
The addition of SO2 to a culture of S. cerevisiae induced entry into a VBNC state with a significant decrease of the metabolic activity. According to literature, the removal of the stressor factor can induce the exit from the VBNC state. In this study, the removal of molecular SO2 was performed by increasing the pH of the medium. Under these conditions, the ability of the cells to recover culturability after the stress removal was observed.
The green fluorescence detected by FCM using FDA reflected a true metabolic activity which indicates that cells that are considered in VBNC state after being exposed to SO2 stress and still detectable by FCM are metabolically active in a VBNC state (Fig. 2). This was further validated by the observation of CIVS structures, detected by FUN-1 probe. As the formation of these structures is strongly dependent on ATP, this further demonstrated the presence of metabolic activity.
We report that yeast cells can survive in a VBNC state in synthetic wine for up to one month. It is likely that Saccharomyces yeast cells could even stay longer in this state. The specific molecular mechanism involved in the entry into and exit from the VBNC state remains to be unraveled. A transcriptomic approach of VBNC cells would be useful to assess the existence of such a mechanism. From a practical point of view, this result demonstrates that the use of sulfite for stabilizing different beverages should be assessed using other methods than plating methods.
Supporting Information
Effect of the increasing pH on the growth dynamic of a culture of S. cerevisiae S288C. Total cell counts (♦), culturable counts (▪), and viable counts (▴) are shown. pH increased at 3 days (72 h). The values presented are the average of three replicates of three separate experiments.
(TIFF)
FCM histograms of S. cerevisiae S288C cells stained with FDA. The cells were incubated with 10 g/L SO2 in Synthetic wine at 28°C. After 15 (B); 30(C); 45(D) and 60(E) min, the cells were collected, and the cell Green fluorescence intensity was analyzed by FCM, Panel A represents control cells in the absence of SO2 (0 min). The Green fluorescence intensity (GRN-HLog) is represented on the x-axis, and cell counts are represented on the y-axis. Panels show the fluorescence of S. cerevisiae S288C before (red arrow; self-fluorescence) and after (blue arrow) staining with FDA. One representative experiment of the three performed is shown.
(TIFF)
Funding Statement
This research was funded by the French Minister of Research. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Helmann JD (2002) The extracytoplasmic function (ECF) sigma factors. Adv Microb Physiol 46: 47–110. [DOI] [PubMed] [Google Scholar]
- 2.Yeast Stress Responses (n.d.). Available: http://www.springer.com/lifesciences/microbiology/book/978-3-540-43926-4. Accessed 16 May 2013.
- 3. Giraffa G (2004) Studying the dynamics of microbial populations during food fermentation. FEMS Microbiol Rev 28: 251–260. [DOI] [PubMed] [Google Scholar]
- 4. Kell DB, Kaprelyants AS, Weichart DH, Harwood CR, Barer MR (1998) Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Van Leeuwenhoek 73: 169–187. [DOI] [PubMed] [Google Scholar]
- 5. Yamamoto H (2000) Viable but nonculturable state as a general phenomenon of non-spore-forming bacteria, and its modeling. J Infect Chemother 6: 112–114. [DOI] [PubMed] [Google Scholar]
- 6. Xu H-S, Roberts N, Singleton FL, Attwell RW, Grimes DJ, et al. (1982) Survival and viability of nonculturable Escherichia coli and Vibrio cholerae in the estuarine and marine environment. Microb Ecol 8: 313–323 10.1007/BF02010671 [DOI] [PubMed] [Google Scholar]
- 7. Oliver JD, Hite F, McDougald D, Andon NL, Simpson LM (1995) Entry into, and resuscitation from, the viable but nonculturable state by Vibrio vulnificus in an estuarine environment. Appl Environ Microbiol 61: 2624–2630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Roth WG, Leckie MP, Dietzler DN (1988) Restoration of colony-forming activity in osmotically stressed Escherichia coli by betaine. Appl Environ Microbiol 54: 3142–3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gauthier MJ (2000) Environmental Parameters Associated with the Viable but Nonculturable State. In: Colwell RR, Grimes DJ, editors. Nonculturable Microorganisms in the Environment. Springer US. 87–112. Accessed 16 May 2013.
- 10. Rollins DM, Colwell RR (1986) Viable but nonculturable stage of Campylobacter jejuni and its role in survival in the natural aquatic environment. Appl Environ Microbiol 52: 531–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver JD (2005) The viable but nonculturable state in bacteria. J Microbiol Seoul Korea 43 Spec No: 93–100. [PubMed]
- 12. Del Mar Lleo M, Pierobon S, Tafi MC, Signoretto C, Canepari P (2000) mRNA Detection by Reverse Transcription-PCR for Monitoring Viability over Time in an Enterococcus faecalis Viable but Nonculturable Population Maintained in a Laboratory Microcosm. Appl Environ Microbiol 66: 4564–4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Millet V (2001) Dynamique et survie des populations bactériennes dans les vins rouges au cours de l’élevage: interactions et équilibres [thesis]. Available: http://babordplus.univ-bordeaux.fr/notice.php?q=id:351964. Accessed 16 May 2013.
- 14. Guillou S, Besnard V, El Murr N, Federighi M (2003) Viability of Saccharomyces cerevisiae cells exposed to low-amperage electrolysis as assessed by staining procedure and ATP content. Int J Food Microbiol 88: 85–89. [DOI] [PubMed] [Google Scholar]
- 15. Bleve G, Rizzotti L, Dellaglio F, Torriani S (2003) Development of reverse transcription (RT)-PCR and real-time RT-PCR assays for rapid detection and quantification of viable yeasts and molds contaminating yogurts and pasteurized food products. Appl Environ Microbiol 69: 4116–4122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mills DA, Johannsen EA, Cocolin L (2002) Yeast Diversity and Persistence in Botrytis-Affected Wine Fermentations. Appl Environ Microbiol 68: 4884–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Serpaggi V, Remize F, Recorbet G, Gaudot-Dumas E, Sequeira-Le Grand A, et al. (2012) Characterization of the “viable but nonculturable” (VBNC) state in the wine spoilage yeast Brettanomyces . Food Microbiol 30: 438–447. [DOI] [PubMed] [Google Scholar]
- 18. Du Toit WJ, Pretorius IS, Lonvaud-Funel A (2005) The effect of sulphur dioxide and oxygen on the viability and culturability of a strain of Acetobacter pasteurianus and a strain of Brettanomyces bruxellensis isolated from wine. J Appl Microbiol 98: 862–871. [DOI] [PubMed] [Google Scholar]
- 19. Agnolucci M, Rea F, Sbrana C, Cristani C, Fracassetti D, et al. (2010) Sulphur dioxide affects culturability and volatile phenol production by Brettanomyces/Dekkera bruxellensis . Int J Food Microbiol 143: 76–80. [DOI] [PubMed] [Google Scholar]
- 20. Divol B, Lonvaud-Funel A (2005) Evidence for viable but nonculturable yeasts in botrytis-affected wine. J Appl Microbiol 99: 85–93. [DOI] [PubMed] [Google Scholar]
- 21. Bogosian G, Bourneuf EV (2001) A matter of bacterial life and death. EMBO Rep 2: 770–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Roszak DB, Grimes DJ, Colwell RR (1984) Viable but nonrecoverable stage of Salmonella enteritidis in aquatic systems. Can J Microbiol 30: 334–338. [DOI] [PubMed] [Google Scholar]
- 23. Nilsson L, Oliver JD, Kjelleberg S (1991) Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol 173: 5054–5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ravel J, Knight IT, Monahan CE, Hill RT, Colwell RR (1995) Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiol Read Engl 141 (Pt 2): 377–383. [DOI] [PubMed] [Google Scholar]
- 25. McDougald D, Rice SA, Weichart D, Kjelleberg S (1998) Nonculturability: adaptation or debilitation? FEMS Microbiol Ecol 25: 1–9. [Google Scholar]
- 26.Ausubel FM (1999) Short protocols in molecular biology: a compendium of methods from Current protocols in molecular biology. 4th ed. New York: Wiley. 1 p. [Google Scholar]
- 27. Gundllapalli SB, Otero RRC, Pretorius IS (2006) Development of a screening method for the indentification of a novel Saccharomyces cerevisiae mutant over-expressing Trichoderma reesei cellobiohydrolase II. Ann Microbiol 56: 143–150. [Google Scholar]
- 28.Sambrook J, Fritsch EF, Maniatis T (1989) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory. book p.
- 29. Volschenk H, Bloom.M, Van StadenJ, Husnik.J, Van VuurenHjj (2004) Genetic engineering of an industrial strain of Saccharomyces cerevisiae for L-Malic acid degradation via an efficient malo-ethanolic. Vol. 25 (2): p.63–72. [Google Scholar]
- 30. Millard PJ, Roth BL, Thi HP, Yue ST, Haugland RP (1997) Development of the FUN-1 family of fluorescent probes for vacuole labeling and viability testing of yeasts. Appl Environ Microbiol 63: 2897–2905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Romano P, Suzzi G (1993) Wine: Microbiology and Biotechnology. Graham H. Fleet, editor Taylor & Francis. 524 p. [Google Scholar]
- 32.Singleton VL, Bisson LF (1998) Principles and Practices of Winemaking. Springer. 632 p. [Google Scholar]
- 33. Davey HM, Kell DB (1996) Flow cytometry and cell sorting of heterogeneous microbial populations: the importance of single-cell analyses. Microbiol Rev 60: 641–696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Teixeira H, Gonçalves MG, Rozès N, Ramos A, San Romão MV (2002) Lactobacillic acid accumulation in the plasma membrane of Oenococcus oeni: a response to ethanol stress? Microb Ecol 43: 146–153. [DOI] [PubMed] [Google Scholar]
- 35. Pozarowski P, Darzynkiewicz Z (2004) Analysis of cell cycle by flow cytometry. Methods Mol Biol Clifton NJ 281: 301–311 10.1385/1-59259-811-0:301 [DOI] [PubMed] [Google Scholar]
- 36. Minois N, Lagona F, Frajnt M, Vaupel JW (2009) Plasticity of death rates in stationary phase in Saccharomyces cerevisiae . Aging Cell 8: 36–44. [DOI] [PubMed] [Google Scholar]
- 37. Schimz K-L (1980) The effect of sulfite on the yeast Saccharomyces cerevisiae . Arch Microbiol 125: 89–95. [DOI] [PubMed] [Google Scholar]
- 38. Bogosian G, Morris PJL, O’Neil JP (1998) A Mixed Culture Recovery Method Indicates that Enteric Bacteria Do Not Enter the Viable but Nonculturable State. Appl Environ Microbiol 64: 1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Nyström T (2003) Nonculturable bacteria: programmed survival forms or cells at death’s door? BioEssays News Rev Mol Cell Dev Biol 25: 204–211. [DOI] [PubMed] [Google Scholar]
- 40. Dhiaf A, Bakhrouf A, Witzel K-P (2008) Resuscitation of eleven-year VBNC Citrobacter . J Water Health 6: 565–568 10.2166/wh.2008.131 [DOI] [PubMed] [Google Scholar]
- 41. Zhong L, Chen J, Zhang X, Jiang Y (2009) Entry of Vibrio cincinnatiensis into viable but nonculturable state and its resuscitation. Lett Appl Microbiol 48: 247–252. [DOI] [PubMed] [Google Scholar]
- 42. Park H, Hwang Y-S (2008) Genome-wide transcriptional responses to sulfite in Saccharomyces cerevisiae . J Microbiol Seoul Korea 46: 542–548 10.1007/s12275-008-0053-y [DOI] [PubMed] [Google Scholar]
- 43. Casalone E, Colella CM, Daly S, Gallori E, Moriani L, et al. (1992) Mechanism of resistance to sulphite in Saccharomyces cerevisiae . Curr Genet 22: 435–440. [DOI] [PubMed] [Google Scholar]
- 44. Avram D, Bakalinsky AT (1997) SSU1 encodes a plasma membrane protein with a central role in a network of proteins conferring sulfite tolerance in Saccharomyces cerevisiae . J Bacteriol 179: 5971–5974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Park H, Bakalinsky AT (2000) SSU1 mediates sulphite efflux in Saccharomyces cerevisiae . Yeast Chichester Engl 16: 881–888. [DOI] [PubMed] [Google Scholar]
- 46. Brachmann CB, Davies A, Cost GJ, Caputo E, Li J, et al. (1998) Designer deletion strains derived from Saccharomyces cerevisiae S288C: a useful set of strains and plasmids for PCR-mediated gene disruption and other applications. Yeast Chichester Engl 14: 115–132. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Effect of the increasing pH on the growth dynamic of a culture of S. cerevisiae S288C. Total cell counts (♦), culturable counts (▪), and viable counts (▴) are shown. pH increased at 3 days (72 h). The values presented are the average of three replicates of three separate experiments.
(TIFF)
FCM histograms of S. cerevisiae S288C cells stained with FDA. The cells were incubated with 10 g/L SO2 in Synthetic wine at 28°C. After 15 (B); 30(C); 45(D) and 60(E) min, the cells were collected, and the cell Green fluorescence intensity was analyzed by FCM, Panel A represents control cells in the absence of SO2 (0 min). The Green fluorescence intensity (GRN-HLog) is represented on the x-axis, and cell counts are represented on the y-axis. Panels show the fluorescence of S. cerevisiae S288C before (red arrow; self-fluorescence) and after (blue arrow) staining with FDA. One representative experiment of the three performed is shown.
(TIFF)