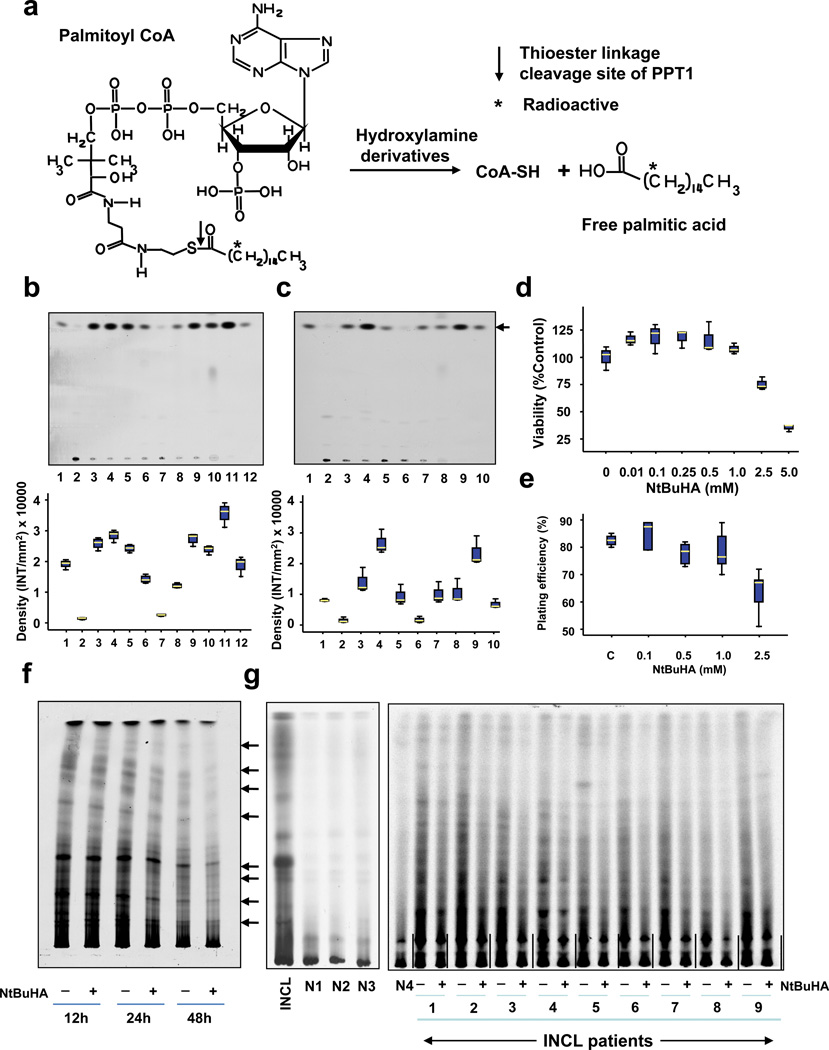
Figure 1.
Hydroxylamine derivatives cleave thioester linkage in [14C]palmitoyl~CoA.
(Panel a) Method of screening of hydroxylamine-derivatives for the cleavage of thioester linkage in [14C]palmitoyl~CoA. Palmitoyl~CoA is a model substrate of PPT1 in which [14C]palmitic acid (asterisk) is attached to CoA via thioester linkage (arrow). PPT1 or nucleophilic compound cleaving the thioester linkage would release radioactive palmitate from [14C]-palmitoyl~CoA. (Panel b) Water-soluble and (Panel c) DMSO-soluble hydroxylamine-derivatives were screened by incubating [14C]palmitoyl~CoA with each of the 12 hydroxylamine-derivatives (Supplementary Table 1) for 1 h at room temperature. Varying degrees of [14C]palmitate were released by both water-soluble (Panel b, lanes 3–9) and DMSO-soluble (Panel c, lanes 3–7) hydroxylamine-derivatives resolved by TLC. (Panel b: lanes: (1): [14C] palmitic acid standard; (2) [14C]-palmitoyl~CoA standard; (3) N-(tert-Butyl)hydroxylamine; (4): N-Benzylhydroxylamine; (5): N-Methylhydroxylamine; (6): N-Cyclohexylhydroxylamine; (7): N,N-Dimethylhydroxylamine; (8): N,N-Diethylhydroxylamine (9): N,O-Bis(trimethylsilyl) hydroxylamine; (10): hydroxylamine (control); (11): recombinant human PPT1 (control); (12): [14C]palmitate standard. (Panel c), lanes: (1): [14C] palmitic acid standard; (2) [14C]-palmitoyl~CoA standard; (3) N-Benzyloxycarbonyl hydroxylamine; (4), N,N-Dibenzylhydroxylamine; (5): N-Benzoyl-N-phenyl hydroxylamine; (6): N-tert-Butyl-O-[1-[4-(chloromethyl) phenyl]ethyl]- N-(2-methyl-1-phenylpropyl) hydroxylamine; (7): N,O-Di-Boc-hydroxylamine; (8): hydroxylamine (control); (9): recombinant human PPT1 (control); (10): [14C]palmitate standard. The arrow indicates the released free [14C] palmitate band. The densitometric quantitation of released free [14C]palmitate bands is represented graphically. (Panel d) Effect of varying concentrations of NtBuHA treatment for 48 h on the viability of cultured lymphoblasts from an INCL patient was evaluated by MTT assay23. (Panel e). Plating efficiency of the NtBuHA-treated INCL fibroblasts compared with that of the normal control cells. Note that compared with the cells treated with up to 1 mM NtBuHA, those treated with 2.5mM NtBuHA manifested slightly lower level of plating efficiency. (Panel f). Time-course of NtBuHA-mediated depletion of [35S]labeled lipid thioester compounds from INCL lymphoblasts. Cultured lymphoblasts from an INCL patient were treated with 500 µM NtBuHA for varying lengths of time (12–48h) after being labeled with [35S]-cysteine. Fresh NtBuHA was added every 12 h. The [35S]-labeled lipid thioesters were extracted and resolved by TLC. Note a marked decrease in intensity of the radioactive bands at 48h. The arrows indicate the positions of each of the 8 identifiable lipid thioester bands (1–8: counting from the top) and the quantitations of these bands are presented graphically (Supplementary Fig. 5a). (Panel g) Depletion of [35S]Cysteine-labeled lipid thioesters in lymphoblasts from INCL patients, which were either untreated or treated with NtBuHA for 48 h. The culture medium was replaced every 12h with fresh NtBuHA containing medium. Because all the normal controls and 9 INCL patients’ cells could not be accommodated in one TLC plate the samples are resolved using two TLC plates. The smaller TLC (left): INCL, INCL patient’s lymphoblast; N1–N3, 3 normal control lymphoblasts; Larger TLC (right) N4, normal lymphoblasts (control), which in addition to N1–N3 (smaller TLC) show virtually no dense thioester bands. However, lymphoblasts from 9 INCL patients (Supplementary Table 2) show clearly identifiable lipid thioester bands. The [35S]labeled lipid thioesters were resolved by TLC as previously reported18. Lanes marked ‘–’ or ‘+’ indicate untreated and NtBuHA-treated samples, respectively. Note the reduction in density of the radioactive lipid thioester bands in NtBuHA-treated samples. Quantitative analyses of each of the 8 identifiable lipid thioester bands marked in panel f were performed and the results are shown in Supplementary Fig.5b.