Abstract
Objective
To evaluate if mediational intervention for sensitizing caregivers (MISC) MISC biweekly caregiver training significantly enhanced child development, compared with biweekly training on health and nutrition (active control) and to evaluate whether MISC training improved the emotional well-being of the caregivers compared with controls.
Study design
Sixty of 120 rural Ugandan preschool child/caregiver dyads with HIV were assigned by randomized clusters to biweekly MISC training alternating between home and clinic for one year. Control dyads received a health and nutrition curriculum. Children were evaluated at baseline, 6 months, and 1 year with the Mullen Early Learning Scales and the Color-Object Association Test (COAT) for memory. Caldwell HOME and videotaped child/caregiver MISC interactions also were evaluated. Caregivers were evaluated for depression and anxiety with the Hopkins Symptoms Checklist.
Results
Between-group repeated-measures ANCOVA comparisons were made with age, sex, CD4 levels, viral load, material SES, physical development, and HAART treatment status as covariates. The children given MISC had significantly greater gains compared with controls on the Mullen Visual Reception scale (visual-spatial memory) and on COAT memory. MISC caregivers significantly improved on HOME scale and total frequency of MISC videotaped interactions. MISC caregivers also were less depressed. Mortality was less for children given MISC compared with controls during the training year.
Conclusions
MISC was effective in teaching Ugandan caregivers to enhance their children’s cognitive development through practical and sustainable techniques applied during daily interactions in the home.
Keywords: child development, HIV, caregiver, training, Uganda, language, HAART, nutrition, cognition
Enhanced access to highly active anti-retroviral therapy (HAART) medications for children in the developing world has changed the prognosis for infected children from a uniformly deadly disease early in childhood, to one in which survival well into adolescence is not uncommon.(1) African children with HIV are now able to survive longer, but they remain at significant risk developmentally, partly because of psychosocial distress from compromised caregiving and HIV-related orphanhood.(2) Therefore, it is important to consider strategies for enhancing their cognitive and psychosocial development in the face of HIV disease encephalopathy, psychosocial distress, malnutrition, and seriously compromised caregiving due to parental illness from HIV/AIDS.(3)
The mediational interaction for sensitizing caregivers (MISC) approach is a training program providing caregivers with strategies for enhancing the development of their children through day-to-day interactions in the home. The MISC approach was developed by Dr Pnina Klein, who has documented the effectiveness of this approach with impoverished children in Africa and globally.(4, 5) Unlike models based on simple direct learning through stimulating the senses with an enriched environment,(6–11) MISC is a mediational approach based on Feurerstein’s theory of cognitive modifiability.(12, 13) We selected MISC for our caregiver training intervention because it is culturally adaptable in low resource settings and significantly enhanced cognitive, language, behavioral, and academic outcomes in impoverished children in Addis Ababa, Ethiopia.(4, 10, 14)
The fundamental premise of this approach is that mediated learning best occurs interactively, when the caregiver interprets the environment for the child. To do so, the caregiver must be sensitive to the child’s cognitive and emotional needs, interests and capacities.
MISC training teaches the caregiver practical strategies for: (1) focusing (gaining the child’s attention and directing him/her to the learning experience in an engaging manner); (2) exciting (communicating emotional excitement, appreciation, and affection with the learning experience); (3) expanding (making the child aware of how that learning experience transcends the present situation and can include past and future needs and issues); (4) encouraging (emotional support of the child to foster a sense of security and competence); and (5) regulating (helping direct and shape the child’s behavior in constructive ways with a goal towards self-regulation).(4, 5, 10, 15)
The principal study goal was to implement a MISC caregiver training intervention with the principal caregivers of preschool-age children with HIV in an impoverished rural district area of Uganda. Using a prospective treatment and active control group cohort design, we hypothesized that a year-long biweekly MISC caregiver training program would significantly enhance the development of these children (motor, cognitive, psychosocial) compared with controls. This is because African children with HIV show poorer scores in memory, attention, language skills, visual processing, reasoning and motor skills.(16–18) The 2nd study aim was to evaluate the impact of the MISC intervention on the emotional well-being of the caregivers themselves. Such benefits were qualitatively observed in the Ethiopia MISC study.(11)
METHOD
IRB approval for this study was obtained by Michigan State University and Makerere University. Research permission was issued by the Ugandan National Council for Science and Technology (UNCST). Children in Kayunga town and surrounding districts, Uganda (80 km northeast of Kampala) were referred to our study by Childhealth Advocacy International (CAI) of Uganda. CAI was a non-government organization (NGO) providing monthly home-based medical care to children with HIV in Kayunga district.(19) We selected this study site because of the opportunity to collaborate with CAI. After consent, 120 caregiver/child dyads were randomly assigned to either biweekly MISC training intervention or a health and nutrition educational curriculum (active control group).
The 60 children given MISC ranged from 16 months to 5 years of age at enrollment (M = 3.8 yrs, SD = 1.2; 58% male) as did the control children (M = 3.5 yrs, SD = 1.4; 51.7 % male). At the time of the study the Uganda Ministry of Health followed the 2006 WHO guidelines (CD4% < 20, < 750 mm3 for children 12 to 36 months) for determining when to initiate highly active anti-retroviral therapy (HAART) in children with HIV. The Walter Reed Project in Kayunga district managed the clinical care of children with HIV in Kayunga through United States President’s Emergency Plan for AIDS Relief (PEPFAR) support. One-half of the children given MISC and 57.8 % of the control children were on HAART treatment during the intervention year (Trimune: d4T/3TC/Nevirapine). CD4 and CD8 activation measures in Table I are presented as percentages of CD38, HLA-DR. Biweekly training sessions with both groups of caregivers were an hour long and alternated between home (so trainer could observe and direct caregiver/child interactions) and study office (where videotapes of interactions were used).
Table 1.
Baseline mean (M) and standard deviation (SD) measures at enrollment (baseline) with corresponding between-group difference P value for the two intervention groups: mediational intervention for sensitizing caregivers (MISC) training and the UCOBAC health/nutrition education curriculum (Control) intervention.
| Descriptive Measures | MISC (N = 60) | Control (N = 60) | P value |
|---|---|---|---|
| M (SD) | M (SD) | ||
|
| |||
| Child Age, yrs | 3.8 (1.2) | 3.5 (1.4) | 0.261 |
| Child sex -male, n (%) | 34 (56.7%) | 31 (51.7%) | 0.512 |
| Primary Caregiver –biological mother, n (%) | 32 (53.3%) | 39 (65.0%) | 0.412 |
| Percentage on HAART treatment | 30 (50.0%) | 34 (56.7%) | 0.442 |
| CD4% | 37.5 (12.5) | 34.4 (14.90) | 0.26 |
| Log plasma HIV RNA level, copies/mL | 8.5 (4.4) | 10.1 (3.4) | 0.05 |
| CD4/CD38 HLA-DR percent activation | 9.3 (5.3) | 11.5 (11.1) | 0.19 |
| CD8/CD38 HLA-DR percent activation | 22.8 (13.4) | 23.4 (12.3) | 0.83 |
| Child weight, kgs | 12.8 (2.95) | 12.1 (3.12) | 0.21 |
| Child height, cm | 92.2 (9.80) | 88.2 (10.11) | 0.04 |
| Child mid-upper-arm circumference, cm | 15.5 (2.74) | 15.2 (1.58) | 0.32 |
| Child weight-for-age Z# | −2.19 (2.12) | −2.21 (1.66) | 0.93 |
| Socio-economic status (material possessions) | 3.4 (2.80) | 3.1 (1.90) | 0.08 |
| Caldwell HOME total score | 20.3 (2.61) | 19.3 (2.90) | 0.05 |
| Gross Motor T scorea | 32.8 (11.56) | 29.7 (10.33) | 0.44 |
| Fine Motor T scorea | 32.4 (13.44) | 33.1 (12.11) | 0.79 |
| Visual Reception T scorea | 30.7 (11.91) | 29.5 (11.51) | 0.60 |
| Receptive Language T scorea | 36.6 (11.25) | 33.7 (11.57) | 0.19 |
| Expressive Language T scorea | 39.8 (16.27) | 35.2 (13.12) | 0.09 |
| MELS Composite Standard scorea | 72.6 (20.31) | 69.3 (16.52) | 0.33 |
| COAT Immediate Memory raw scoreb | 3.8 (3.29) | 3.6 (3.62) | 0.72 |
| COAT Total Memory raw scoreb | 8.0 (7.59) | 6.7 (8.30) | 0.38 |
| CBCL Internalising problems T scorec | 63.0 (9.79) | 64.5 (9.21) | 0.39 |
| CBCL Externalising problems T scorec | 53.8 (7.42) | 55.0 (7.64) | 0.38 |
| CBCL Total Problems T scorec | 59.1 (7.74) | 62.5 (9.36) | 0.03 |
| HSCL-25 Depression raw scored | 11.5 (7.10) | 13.8 (8.60) | 0.30 |
| HSCL-25 Anxiety raw scored | 7.8 (6.00) | 7.7 (6.50) | 0.95 |
Using CDC 2000 norms (Epi Info)
Mullen Early Learning Scale (MELS) T values (adjusted for age and gender using American norms)
Color-Object Association Test (COAT)
Achenbach Child Behavior Checklist (CBCL) parent form for children 1.5 to 5 yrs T scores (adjusted for age and gender using Cross-Cultural Group 2 norms)
Hopkins Symptoms Checklist 25-item (HSCL-25) scale for caregiver depression and anxiety
all P values are based on Student t test for two independent samples, unless otherwise noted.
P value based on Chi-square test for categorical relationships.
Children were included in the study if they had HIV at age 16 months to 5 years, born to a mother with confirmed HIV.
Children were excluded from the study if they had a medical history of serious birth complications, severe malnutrition, bacterial meningitis, encephalitis, cerebral malaria, or other known brain injury or disorder requiring hospitalization and which could overshadow the developmental benefits of MISC. Children enrolled in school during the training year were excluded because caregivers with children still at home would benefit most from caregiver MISC training.
Prior to and during the study, our MISC consultants (PK, CS, DG) did several week-long workshops with our Ugandan field team on how to train caregivers in the MISC program. The program director for the Uganda Community Based Organization for Child Welfare (UCOBAC; http://ucobac.org/cms/) trained a separate field team for training the control-group caregivers in a health/nutrition educational program. UCOBAC had implemented this curriculum in impoverished households in 17 other rural Ugandan districts.
The MISC consultants also taught the field team how to videotape 5 minute segments of the caregiver bathing the child, feeding the child, and working with the child. These 15-min recordings were made for the MISC intervention dyads in the home at the start of the year-long training and thereafter every three months. The tapes were played back to the caregivers by the field trainers and used as part of the MISC training during each biweekly session. The video recordings at baseline, 6 months, and 1 year were scored by an independent observer at a separate study site, using the Observing Mediated Interactions (OMI) rubric developed by Dr Klein.(4, 5, 10, 15) OMI scoring of 15 minute video tapes were scored for the total number of focusing, exciting, expanding, encouraging, and regulating MISC-type caregiver/child interactions. This measure helped document fidelity of training and served as one of our training outcomes. Similar recordings were also scored for the control group, but not used in their biweekly training.
At baseline, 6 months, and one year a home visitor independently administered the toddler version of the Caldwell Home Observation for Measurement of the Environment (HOME) scale.(20) We wanted a measure of caregiving quality that was independent of the OMI, and we have previously adapted the HOME in the Ugandan context and it has proven sensitive to cognitive outcomes in children.(21) We also included an SES measure based on dwelling (e.g., roofing and flooring composition, number in household, water source, toilet facilities, and cooking facilities) and material possessions (e.g., farm animals, bicycle, radio, furnishings, and appliances).
Developmental Outcomes
At baseline, six months, and 1 year testers blinded to the intervention group assessed study children with the Mullen Early Learning Scales (MELS) in a private quiet setting at our study office. Testing was done in the local language of Luganda. The MELS has scales in motor, language, and overall cognitive skills development. The MELS has excellent correspondence validity with the Bayley Scales of Infant Development, yet is designed to be easier to implement in cross-cultural settings.(22) The MELS had been previously adapted for use with children with HIV in rural Uganda, and had proven sensitive to the developmental benefits of early initiation of anti-retroviral treatment and quality of home environment (ART).(23)
Children were also assessed with the Color-Object Association Test (COAT) for memory. This is an experimental test that uses the placement of small toys inside of small colored boxes to test visual memory, and is not dependent on a given language competency.(24) It was chosen to assess immediate memory for objects and a total memory score (that includes delayed recall).
Although the child was undergoing developmental assessment, the principal caregiver was interviewed in Luganda with the Achenbach Child Behavior Checklist (CBCL) for younger children (1.5 to 5 yrs).(25, 26) The CBCL is a psychiatric screening for internalizing symptoms (emotional), externalizing symptoms (behavioral), and total symptoms that has been previously validated in the Uganda setting following translation and back-translation.(27)
Anthropometric and immunology measurements
At enrollment the child’s height, weight, and mid-upper arm circumference were measured. Blood draws were also taken for CD4 and CD8 percent and activation measures along with viral load. These were performed at the Makerere University – Walter Reed laboratory at Mulago Hospital in Kampala. HAART treatment status for the study children was also noted from the Walter Reed Project HIV clinic at Kayunga District Hospital.
Hopkins Symptom Checklist (HSCL)
The HSCL is a 25-item scale previously used to assess depression and anxiety among adults in HIV-affected Ugandan communities.(28) This questionnaire was given to the caregiver in Luganda during the home evaluation visit at 6 and 12 months, providing an emotional wellbeing outcome for comparing the MISC and control caregivers.
Statistical Analyses
We used a repeated-measure (baseline, 6, and 12 mos.) ANCOVA (RM-ANCOVA) in SPSS to evaluate MELS developmental and COAT memory outcomes in comparing the MISC and control children. In this analysis, the group (MISC, Control) by time (baseline, 6, and 12 mos.) interaction indicates a significant outcome difference between the two interventions. The group-by-time effects were adjusted for age, sex, physical development (standardized anthropometric measures using Epi Info 2010 WHO norms), caregiver category (mother, grandmother, other), HAART treatment status, and SES. Some of the models are additive and some have one interaction if an interaction effect was significant. RM-ANCOVA was also used with quality of caregiving outcomes (HOME, OMI video scores). A two-factor additive ANOVA (MISC and Control group, caregiver category) was used to compare caregivers on HSCL depression and anxiety scores at 6 and 12 months, because these caregiver measures were not available at baseline in this study.
RESULTS
For those caregivers completing the entire year of training, thirty-two of the 55 MISC caregivers were the biological mother (56 %), 16 were the grandmother (29 %), and the remaining 8 (14.5%) were the father, aunt, step mother, or older sibling (Table I for baseline demographics). Twenty-seven of the 45 control caregivers were the mother (60%), 10 were the grandmother (22%), and 8 were “others” (18%) (Chi Square (2) = 1.81, P = 0.41). At baseline, the control children had higher viral loads (t (117) = 1.53, P = 0.05).
Two of the 60 children given MISC and 10 of the 60 control children died from opportunistic infections. This represented a significant relationship between mortality and treatment group (P = 0.01). Two of the 60 MISC caregivers withdrew from the study, and 5 of the control-group children were lost to the study due to re-location of caregivers. The overall proportion of control caregiver/child dyads lost to intervention and follow-up during the year was significantly greater than for the MISC dyads (Table I; Chi Square (1) = 7.82, P = 0.006).
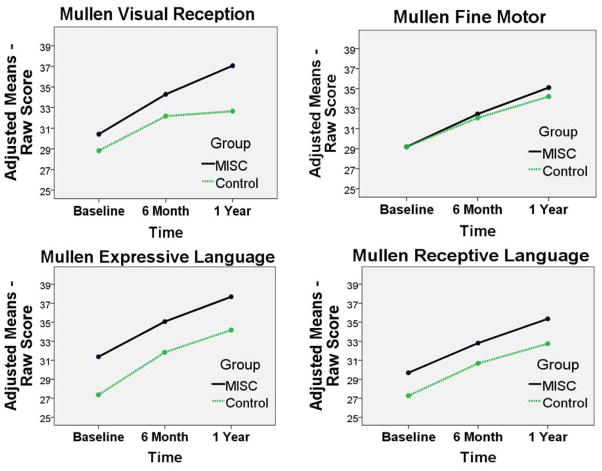
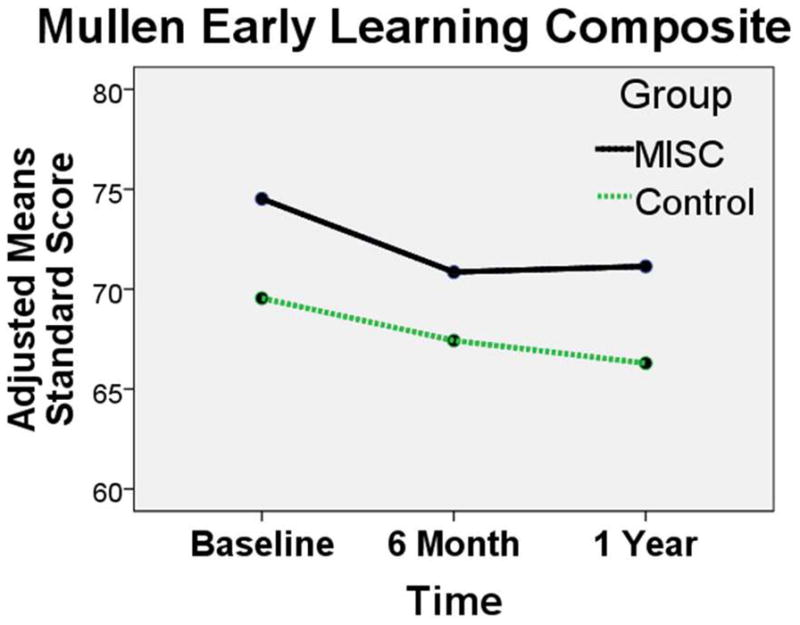
In the RM-ANCOVA analysis for the MELS scale measures, the children given MISC were higher as a group overall on all the MELS measures (Figures 1 and 2 and Table II; available at www.jpeds.com)). However, the children given MISC showed greater gains than controls over time (group-by-time) only on the MELS Visual Reception scale (F (2) = 4.40, P = 0.014, observed power = 0.753); and the unadjusted effect for the composite score (combining the cognitive scales of Visual Reception, Fine Motor, Receptive Language, and Expressive Language) (F (2) = 3.39, P = 0.07, observed power = 0.44). The MELS composite score group-by-time effect was not significant for the adjusted F value (Table II).
Figure 1.
Adjusted group mean score across the three time points (baseline, 6 months, 1 yr for the MISC (black, solid line) and control (green, dash line) caregiver training intervention groups. The means are adjusted according to the RM-ANCOVA model presented in Table II. The group (MISC, control) by assessment (baseline, 6 month, 1 year) adjusted mean plots are presented for the four Mullen cognitive scales (Fine Motor, Visual Reception, Expressive Language, Receptive Language).
Figure 2.
Adjusted group mean score across the three time points (baseline, 6 months, 1 yr for the MISC (black, solid line) and control (green, dash line) caregiver training intervention groups. The means are adjusted according to the RM-ANCOVA model presented in Table II. The group (MISC, control) by assessment (baseline, 6 month, 1 year) adjusted mean plots are presented for the Mullen Composite Total score for the four cognitive scales (Fine Motor, Visual Reception, Expressive Language, Receptive Language).
Table 2. Effect of caregiver training program on.
Mullen Early Learning Scales (MELS) Outcomes.
| Mullen Early Learning Scale | Gross Motor | Fine Motor | Expressive Language | Receptive Language | Visual Reception | Composite Total (Cognitive Scales) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor or Covariate Measure | Unadj | One- Interaction | Unadj | Additive | Unadj | Additive | Unadj | One- Interaction | Unadj | Additive | Unadj | Additive |
| Group-by-time | .611 | .431 | .342 | .341 | .880 | .847 | .565 | .888 | .006** | .014* | .070 | .194 |
| Group Main Effect | .041 | .102 | .767 | .979 | .028* | .046* | .022* | .011* | .036* | .024* | .033* | .038* |
| Caregiver Category | .106 | .362 | .120 | .089 | .019* | .043* | ||||||
| On HAART or Not | .038* | .081 | .523 | .144 | .316 | .231 | ||||||
| Age | .001# | .001# | .001# | .001# | .001# | .001# | .001# | .001# | .001# | .001# | .001# | .001# |
| Caldwell HOME total | .583 | .069 | .523 | .046* | .019* | .072 | ||||||
| SES Total | .668 | .326 | .079 | .081 | .046* | .048* | ||||||
| Weight-for-Age Z | .001** | .001# | .001# | .001** | .001# | .001# | ||||||
| HSCL-25 Caregiver Depression 6 months | .225 | .351 | .626 | .906 | .607 | .757 | ||||||
| Sex | .121 | .723 | .260 | .976 | .340 | .867 | ||||||
| Group by sex | .028* | |||||||||||
| Group by On HAART | .009** | |||||||||||
P < .05,
P < .01,
P < .001
Repeated-measure (baseline, 6 mos, 1 yr) analysis of covariance (RM-ANCOVA) was used. For each MELS measure, the raw score was used in the analysis. Results are presented for the unadjusted model (Unadj) and either the RM-ANCOVA model with one significant interaction (One Interaction) or an additive model if there were no significant interaction effects. The ANCOVA adjusted P values are presented for the between-group factor (Group: MISC and Control) and Group-by-time (baseline, 6 months, 1 year). Covariates in the model are caregiver category (mother, grandmother, other), HAART status (On HAART or not), age at baseline, Caldwell HOME scale score at baseline, SES total at baseline, Weight-for-age Z score (WHO 2010 norms), Hopkins Symptoms Checklist 25-item scale (HSCL-25) depression score for the caregiver at 6 months, and gender.
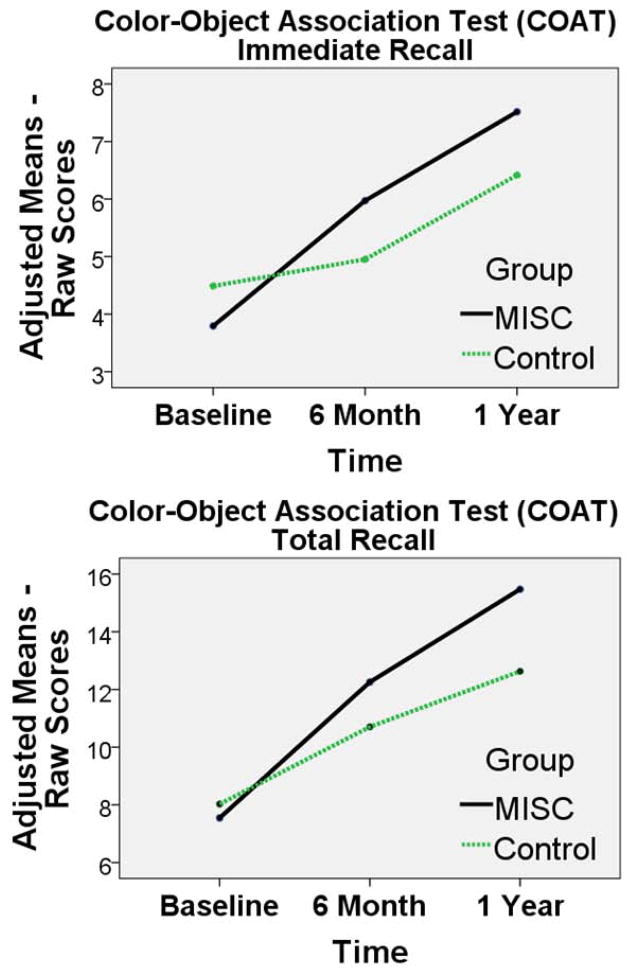
In the RM-ANCOVA analysis for the COAT memory measures, there was a significant group-by-time effect for immediate recall (Figure 3; available at www.jpeds.com) (memory; F (1) = 3.82, P = 0.024, observed power = 0.69). However, there was not a significant group-by-time effect for COAT total memory (F (1) = 2.31, P = 0.103, observed power = 0.46) (Table III).
Figure 3.
Adjusted group mean score across the three time points (baseline, 6 months, 1 year) for the MISC (black, solid line) and Control (green, dash line) caregiver training intervention groups. The means are adjusted according to the RM-ANCOVA model presented in Table III. Both Color-Object Association Test (COAT) scores are presented: Immediate Recall (memory) and Total Recall (learning).
Table 3.
Effect of caregiver training program on Object Memory Global Outcome Measures,
| Color-Object Association Test | Immediate Recall | Total Recall | ||
|---|---|---|---|---|
| Factor or Covariate Measure | Unadjusted | One-Interaction | Unadjusted | One-Interaction |
| Group-by-time | .213 | .024* | .504 | .103 |
| Group Main Effect | .710 | .200 | .590 | .073 |
| Caregiver Category | 586 | .961 | ||
| On HAART or Not | .585 | .767 | ||
| Age | .001# | .001# | .001# | .001# |
| Caldwell HOME total | .821 | .877 | ||
| SES Total | .162 | .025 | ||
| Weight-for-Age Z | .124 | .015* | ||
| HSCL-25 Caregiver Depression 6 months | .694 | .631 | ||
| Sex | .505 | .442 | ||
| Group by Caregiver | .092 | .018* | ||
P < .05,
P < .01,
P < .001
From a repeated-measure (baseline, 6 months, 1 year) analysis of covariance (RM-ANCOVA) was used. For both Color-Object Association Test (COAT) measures (Immediate Recall and Total Recall), the raw score was used in the analysis. Results are presented for the unadjusted model (Unadjusted) and the RM-ANCOVA model with one significant interaction (One-Interaction). The ANCOVA adjusted P values are presented for the between-group factor (Group: MISC and Control) and Group-by-time (baseline, 6 months, 1 year). Covariates in the model are caregiver category (mother, grandmother, other), HAART status (On HAART or not), age at baseline, Caldwell HOME scale score at baseline, SES total at baseline, weight-for-age Z score (WHO 2010 norms), Hopkins Symptoms Checklist 25-item scale (HSCL-25) depression score for the caregiver at 6 months and sex.
In terms of our covariates, children receiving HAART were significantly below those not receiving HAART on a number of developmental outcomes, but significantly so only on MELS Gross Motor development (F (1) = 4.45, P = 0.038, observed power = 0.55). Weight-for-Age z scores (WAZ) was significantly predictive of all MELS scale measures (Table II; WAZ, P < 0.001). Children being cared for by the grandmother or other relatives tended to have higher Mullen developmental outcomes on Visual reception (P = 0.019) and on the Composite score (P = 0.043) than those being cared for by the biological mother (Table II; Caregiver Category covariate). Likewise, the COAT memory outcomes for MISC compared with controls were higher for children being cared for by grandmothers and other relatives, compared with those being cared for by the biological mother (P = 0.018; Table III, Caregiver by Group).
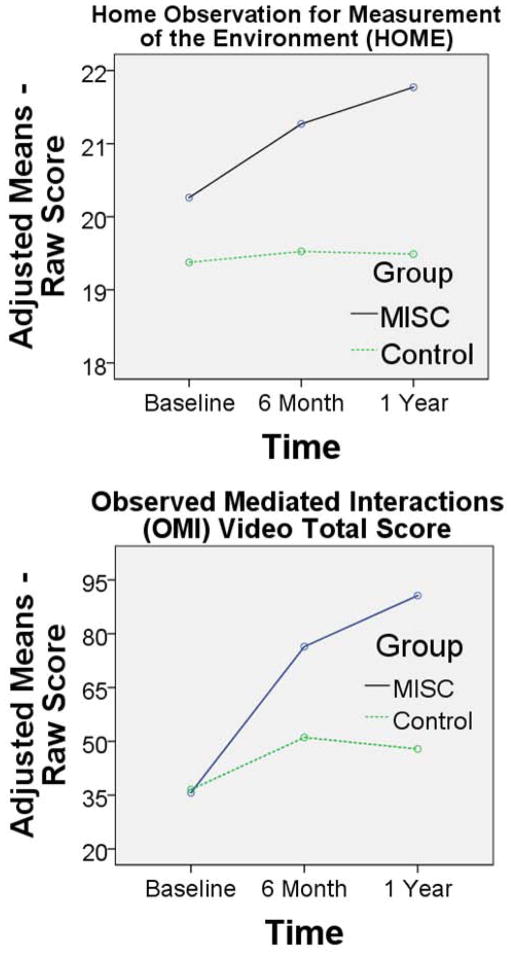
In the RM-ANCOVA analysis for Caldwell HOME and OMI total (video scoring) quality of caregiving total scores, the group-by-time effect was highly significant for both (HOME F (1) = 2.17, P = 0.029, observed power = 0.44; OMI F (1) = 44.87, P < 0.001, observed power = > 0.99) (Table IV and Figure 4; Figure 4 available at www.jpeds.com). Neither the group-by-time or group main effects were significant for the Achenbach CBCL internalizing, externalizing, or total psychiatric symptoms (Table V; available at www.jpeds.com).
Table 4. Effect of Caregiver Training Program on.
Quality of Caregiving Global Outcome Measures
| Quality of Caregiving Measures | Caldwell HOME Total Score | Observed Mediated Interactions (OMI) Video Total Score | ||
|---|---|---|---|---|
| Factor or Covariate Measure | Unadjusted | Additive | Unadjusted | Additive |
| Group-by-time | .078 | .117 | .001# | .001# |
| Group Main Effect | .001# | .001# | .001# | .001# |
| Caregiver Category | .051 | .696 | ||
| On HAART or Not | .144 | .780 | ||
| Age | .871 | .415 | .001# | .001# |
| Caldwell HOME total | .012* | |||
| SES Total | .111 | .845 | ||
| Weight-for-Age Z | .645 | .210 | ||
| HSCL-25 Caregiver Depression 6 mos | .938 | .174 | ||
| Gender | .176 | .546 | ||
P < .05,
P < .01,
P < .001
Repeated-measure (baseline, 6 months, 1 year) analysis of covariance (RM-ANCOVA) was used. For both quality of caregiving outcome measures (Caldwell HOME total score and OMI total score), the raw score is used in the analysis. Results are presented for the unadjusted model (Unadjusted) and the RM-ANCOVA additive model. The ANCOVA adjusted P values are presented for the between-group factor (Group: MISC and Control) and Group-by-time (baseline, 6 months, 1 year). Covariates in the model are caregiver category (mother, grandmother, other), HAART status (On HAART or not), age at baseline, Caldwell HOME scale score at baseline, SES total at baseline (for OMI), weight-for-age Z score (WHO 2010 norms), Hopkins Symptoms Checklist 25-item scale (HSCL-25) depression score for the caregiver at 6 months, and sex.
Figure 4.
Adjusted group mean score across the three time points (baseline, 6 months, 1 year) for the MISC (black/blue, solid line) and Control (green, dash line) caregiver training intervention groups. The means are adjusted according to the RM-ANCOVA model presented in Table IV. Both quality-of-caregiving outcome measures are presented: Caldwell Home Observation for Measurement of the Environment (HOME) total; and Observed Mediated Interactions (OMI) total score from the 15 minute video evaluation of the caregiver feeding, bathing, and working with the child.
Table 5. Effect of Caregiver Training Program on.
Achenbach Child Behavior Checklist (CBCL) Global Outcome Measures (age-based T scores using American norms),
| Achenbach Child Behavior Checklist (CBCL) | Internalizing Symptoms | Externalizing Symptoms | Total Symptoms | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Factor or Covariate Measure | Unadj | Addit | Regress | Unadj | Addit | Regress | Unadj | Addit | Regress |
| Group-by-time | .845 | .866 | .402 | .273 | .183 | .119 | |||
| Group Main Effect | .360 | .667 | .438 | .792 | .450 | .886 | |||
| Caregiver Category | .039* | .660 | .591 | ||||||
| On HAART or Not | .546 | .386 | .445 | ||||||
| Age | |||||||||
| Caldwell HOME total | .001# | .282 | .016* | ||||||
| SES Total | .054 | .125 | .074 | ||||||
| Weight-for-Age Z | .920 | .165 | .353 | ||||||
| HSCL-25 Caregiver Depression 6 months | .001# | .001# | .018* | .066 | .001# | .002** | |||
| Sex | .466 | .907 | .837 | ||||||
P < .05,
P < .01,
P < .001
Repeated-measure (baseline, 6 months, 1 year) analysis of covariance (RM-ANCOVA) was used. Results are presented for the unadjusted model (Unadjusted), the RM-ANCOVA additive model, and a simple linear regression model between Caregiver depression and CBCL global score. The ANCOVA adjusted P values are presented for the between-group factor (Group: MISC and Control) and Group-by-time (baseline, 6 months, 1 year). Covariates in the model are caregiver category (mother, grandmother, other), HAART status (On HAART or not), Caldwell HOME scale score at baseline, SES total at baseline, weight-for-age Z score (WHO 2010 norms), Hopkins Symptoms Checklist 25-item scale (HSCL-25) depression score for the caregiver at 6 months, and sex.
The MISC caregivers were significantly less depressed on the HSCL at 6 months than the control caregivers, irrespective of caregiver category (mother, grandmother, other) (F (1) = 6.14, P = 0.019, observed power = 0.69). Although at 12 months MISC caregivers were still less depressed than control caregivers, the difference was not significant (P = 0.30). MISC and Control caregivers did not differ on the HSCL anxiety measure at 12 months. It should be noted that the home visitors administering the HSCL questionnaire were not blinded to the intervention group for these caregivers.
Caregiver category (mother, grandmother, other) was significant for the Mullen Visual Reception scale (P = 0.019) and the Mullen Composite Total outcomes (P = 0.043; Table II). Across all three assessments (baseline, 6 months, 1 year), children of the grandmothers and mothers did better than those of the “other” category caregivers. Grandmothers and mothers also had higher HOME scores overall (P = 0.051; Table IV), and their children had a lower number of Achenbach CBCL internalizing symptoms (P = 0.039; Table V). The caregiver category by intervention group (MISC, control) interaction effect was not significant, suggesting that the outcome differences between training groups (MISC, control) did not vary significantly by caregiver category. That is why this interaction effect (Group by caregiver Category) was not included in Tables II–V.
Likewise, there were no significant interaction effects between age of the child and training group (MISC, control) for any of the developmental or caregiving quality outcomes. One sex-by-training group interaction effect, Mullen Gross Motor development, was significant (Table II). MISC boys improving more than did the girls, with no sex difference for the control children (P = 0.028).
DISCUSSION
MISC significantly improved cognitive developmental outcomes in visual-spatial memory and learning compared with controls. In a similar study children exposed to HIV (not infected) of mothers with HIV, a year-long biweekly MISC training program for caregivers also showed significantly greater improvements in overall cognitive and learning performance compared wth controls.(29) However, in contrast to the present findings, we also documented significantly greater Mullen receptive and expressive language scale performance for the children given MISC (29).
The lack of significant language-scale differences between the MISC and control group in the present study may be because of differences in the child/caregiver dyads compared with our study with children exposed to HIV (29). With the HIV-exposed dyads, over 90% of the caregivers were the biological mothers. In the present study just over half of the MISC caregivers were the biological mothers. Also, the children exposed to HIV averaged about a year in age younger than the children given MISC in the present study (2.8 yrs versus 3.8 yrs), and varied much less in terms of the age range. MISC may prove more effective in enhancing language development when the mother is trained and is caring for younger children during more sensitive periods of language formation (1.5 to 3 yrs of age). Unfortunately, the present study was limited in terms of the sample size needed to adequately evaluate the statistical relationship between age of the child and comparative impact of MISC versus control training, especially with the mother as principal caregiver. The range of ages was greater in the present study (1 to 5 yrs) encompassing children at various stages of neurocognitive development. The number of children distributed across this age range was not large enough to detect statistically an optimal age for maximum MISC developmental benefit.
The benefit of less reported depression for the MISC caregivers also is noteworthy, given that the mothers in this study had HIV. However, the fact that both MISC and control caregivers were significantly less depressed at one year following baseline suggests that the social support provided through both training interventions were beneficial in this regard. The improved mortality for MISC children compared with control children also is important. MISC is designed to sensitize caregivers to the needs of their children with HIV in general, perhaps strengthening maternal/child attachment which can be weakened for these Ugandan HIV dyads.(30) Ultimately, this can lead to timelier seeking of medical care when an HIV child is ill, or treatment adherence with the child’s ART medications.
The relationship between nutritional wellbeing as measured by physical development, and the Mullen and COAT developmental outcomes for our study children were evident in this study (relationship between the covariate of weight-for-age and the Mullen scale scores in Table II and COAT total recall score in Table III). Nutritional intervention was not a part of the present study design, only educational training of the caregivers. However, it is an important consideration in caregiving quality for the developmental enhancement of children with HIV in low-resource settings. In rural areas of sub-Sahara Africa, the family must rely upon labor-intensive subsistence agriculture to provide for the nutritional needs of the family. Because of this, maternal HIV disease can severely disrupt the nurturing capacity of the mother for her children, and the food security for the entire family.(31–33)
Chronic nutritional hardship can severely undermine early childhood development,(34–36) and weight-for-age is perhaps the single most powerful predictor of developmental outcomes in African children with HIV.(37, 38) This would be the case even in the present study even among those dyads where the biological mother was not trained as the principal caregiver. This is because the biological mother was responsible for the nutritional needs of the child during gestation and birth, and following birth up until the mother was replaced by the grandmother or other family member as the child’s principal caregiver.
The children in the present study had HIV, and a number of these children died or were lost to training and follow-up during the training year, especially in the control group. The differential mortality between MISC and control groups could have diminished the statistical power needed to detect differences in developmental outcome benefits favoring the children given MISC over the course of the year.
Also, over one-half of the study children were initiated on HAART treatment before or during the study year. HAART treatment was initiated when children had a low enough CD4 count and other evidence of significant immune-deficiency related illness. Clinical instability from HIV disease could have overshadowed differences in developmental gains between children in the MISC and control groups. Likewise, the very strong relationship between weight-for-age z scores and the developmental outcomes (Tables II and III) suggests that nutritional need in the present sample of children also could have overshadowed differences in developmental gains between children in the MISC and control groups.
Children under 5 years of age are in a sensitive period of development where they should sustain a dynamic capacity to benefit from new learning experiences. There is a general consensus from developmental research that adult-child interactions are of central importance in this process.(39) Farah et al observed a relationship between parental nurturance and memory development (30). This relationship was consistent with the animal literature on maternal buffering of stress hormone effects on hippocampal development.(40)
Rao et al observed that parental nurturance at age 4 years predicts the volume of the left hippocampus in adolescence, with warmer and more loving nurturance associated with smaller hippocampal volume (41). However, the association between parental nurturance and hippocampal volume disappears at 8 years of age. They concluded that this supports the existence of a sensitive developmental period for brain maturation, especially around 4 years of age.(41) This was the average age of our study children, and the strongest MISC benefits for the present study children were in the developmental domains of memory (Mullen Visual Reception and COAT immediate recall) and learning (Mullen Composite total). The caregiver provides for secure emotional attachments in a nurturing environment, creating learning experiences that allows a child’s neurocognitive ability to blossom.(13, 42) Effective mediational behaviors by caregivers were found to be significantly related to children’s social-emotional stability and the willingness to explore and learn about the world around them.(12, 13) This premise is foundational to the MISC method.
Our caregiver training intervention is evidence-based and family-oriented and has been specifically adapted for use in low resource settings. Our intervention is designed to enhance both emotional and cognitive development in at-risk children. We also documented the psychotherapeutic value of our intervention for the caregiver. MISC provided a mental health benefit for women affected by HIV in low-resource settings. We also demonstrated that MISC was both feasible and acceptable to the local Ugandan population.(43) As it enhances awareness of the child’s emotional and cognitive needs, medical caregiving for children with HIV may also be enhanced with improved survival for children given MISC. MISC does not rely on outside resources or materials and can be implemented with most children in a variety of contexts where caregiver/child interactions naturally take place.
We have demonstrated that this program can be implemented in the field setting by non-professionals for relatively low cost and still be effective. This is good for future dissemination and sustainability in low- and middle-income countries affected by HIV. MISC is a culture-appropriate and sustainable intervention that can be effectively implemented in low-resource settings with children generally at risk from disease, malnutrition, or neglect. The cultural emphasis on the nurturing of children is a good fit for the MISC, which is a method for sensitizing mothers to the positive aspects of their current childrearing interactions. Similarly to the studies by Klein et al,(4) we conclude that the principles of MISC can be understood by Ugandan caregivers in HIV-affected households, and incorporated into their own childrearing approach.(10, 11)
Acknowledgments
Supported by the National Institutes of Health (R34 MH082663 [PI: M.B.] and RO1 HD070723 [PIs: M.B. and J.B.).
Lyagoba Monica, Agatha Kuteesa, Agnes Nakigozi, Godfrey Nyanj, Janet Obita, Paul Oloya, and Peter Masaba served as research assistants for this study. Dr Margaret Nakakeeto (Director of Child Health Advocacy, Uganda), and Jane Nalubega (Administrative Director) provided access to eligible study children through a home healthcare medical program (sponsored by the Princess Diana Memorial Fund). Makerere University – Walter Reed Project of Uganda provided clinical laboratory services and provided ART treatment through the PEPFAR program for many of our study children.
Elizabeth Schut and Erin Lorencz (Michigan State University [MSU]; supported by MSU College of Human Medicine Summer Research Grant and Department of Graduate Studies Research Enhancement Award) assisted in the clinical and laboratory findings in this study. Raina Vachhani (MSU; sponsored by Campaign for America’s Kids, through an AACAP Summer Medical Student Fellowship, and by a University of Michigan Medical School Summer Biomedical Research Program award) coordinated the administration of the Hopkins Symptoms Checklist to our study caregivers.
Dr Nurit Jaeggerman (Department of Child Education at Bar Ilan Univeristy, Israel) participated in the training of our field team in the MISC intervention. Dr Karen Olness (Department of Pediatrics, Case Western Reserve University) served as co-investigator in our quality of home environment assessments, and her expertise and support in these assessments is greatly appreciated. Alice Kayongo (Services Director, Uganda Community Based Association for Child Welfare [sponsored by UNICEF]) trained our field teams in administering their curriculum for our active control intervention.
Abbreviations
- CAI
Childhealth Advocacy International of Uganda
- CBCL
Achenbach Child Behavior Checklist
- CNS
Central Nervous System
- COAT
Color-Object Association Test
- HAART
Highly Active Anti-Retroviral Therapy
- HOME
Caldwell Home Observation for Measurement of the Environment
- HIV
Human Immunodeficiency Virus
- HSCL-25
Hopkins Symptoms Checklist, 25 items
- IRB
Institutional Review Board
- MELS
Mullen Early Learning Scales of Child Development
- MISC
Mediational Intervention for Sensitizing Caregivers
- NGO
Non-Government Organization
- OMI
Observed Mediated Interactions
- PEPFAR
United States President’s Emergency Plan for AIDS Relief
- RM-ANCOVA
Repeated Measure Analysis of Covariance
- SES
Socio-Economic Status
- control group
treatment as usual
- UCOBAC
Uganda Community Based Organization for Child Welfare
- UNCST
Ugandan National Council for Science and Technology
- WAZ
Weight-for-Age adjusted Z score
- WHO
World Health Organization
Footnotes
The authors declare no conflicts of interest.
Registered with ClinicalTrials.gov: NCT00889395.
Acknowledgments available at www.jpeds.com.
All of the individuals in this acknowledgement have no conflict of interest to report. None of the sponsors for their activities had any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Armstrong F, Willen E, Surgen K. Handbook of Pediatric Psychology. 3. 2003. HIV/AIDS in Children and Adolescents; pp. 359–74. [Google Scholar]
- 2.Nyamukapa CA, Gregson S, Lopman B, Saito S, Watts HJ, Monasch R, et al. HIV-Associated Orphanhood and Children’s Psychosocial Distress: Theoretical Framework Tested With Data From Zimbabwe. American Journal of Public Health. 2008;98:133–41. doi: 10.2105/AJPH.2007.116038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bose S. An examination of adaptive functioning in HIV infected children: Exploring the relationships with HIV disease, neurocognitive functioning, and psychosocial characteristics. ProQuest Information & Learning. 1997 Jul;58(409) AAM9719709. 1997; Dissertation Abstracts International: Section B: The Sciences and Engineering. [Google Scholar]
- 4.Klein P, Rye H. Interaction-Oriented Early Intervention in Ethiopia: the MISC Approach. Infants and Young Children. 2004;17:340–54. [Google Scholar]
- 5.Klein PS, editor. Early Intervention: cross-cultural experiences with a mediational approach. New York, NY: Garland Press; 1996. [Google Scholar]
- 6.Grantham-McGregor S, Cheung YB, Cueto S, Glewwe P, Richter L, Strupp B. Developmental potential in the first 5 years for children in developing countries. Lancet. 2007;369:60–70. doi: 10.1016/S0140-6736(07)60032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Grantham-McGregor S, Schofield W, Harris L. Effect of psychosocial stimulation on mental development of severely malnourished children: an interim report. Pediatrics. 1983;72:239–43. [PubMed] [Google Scholar]
- 8.Grantham-McGregor S, Schofield W, Powell C. Development of severely malnourished children who received psychosocial stimulation: six-year follow-up. Pediatrics. 1987;79:247–54. [PubMed] [Google Scholar]
- 9.Grantham-McGregor S, Stewart ME, Schofield WN. Effect of long-term psychosocial stimulation on mental development of severely malnourished children. Lancet. 1980;2:785–9. doi: 10.1016/s0140-6736(80)90395-5. [DOI] [PubMed] [Google Scholar]
- 10.Klein PS, editor. Seeds of hope: twelve years of early intervention in Africa. Oslo, Norway: unipub forlag; 2001. [Google Scholar]
- 11.Klein PS, Rye H. Interaction-oriented early intervention in Ethiopia: the MISC approach. Infants and Young Children. 2004;17:340–54. [Google Scholar]
- 12.Feuerstein R. The dynamic assessment of retarded performers. New York: University Park Press; 1979. [Google Scholar]
- 13.Feuerstein R. Instrumental enrichment: Redevelopment of cognitive functions of retarded performers. New York: University Park Press; 1980. [Google Scholar]
- 14.Klein P. Early Intervention: Cross-cultural experiences with a mediational approach. New York, NY: Garland Press; 1996. [Google Scholar]
- 15.Klein PS. More Intelligent and Sensitive Child. Ramat-Gan, Israel: Bar-Ilan University; 1985. [Google Scholar]
- 16.Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N. Economic advantage and the cognitive ability of rural children in Zaire. J Psychol. 1996;130:95–107. doi: 10.1080/00223980.1996.9914992. [DOI] [PubMed] [Google Scholar]
- 17.Ruel TD, Boivin MJ, Boal HE, Bangirana P, Charlebois E, Havlir DV, et al. Neurocognitive and motor deficits in HIV-infected Ugandan children with high CD4 cell counts. Clin Infect Dis. 2012;54:1001–9. doi: 10.1093/cid/cir1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Van Rie A, Mupuala A, Dow A. Impact of the HIV/AIDS epidemic on the neurodevelopment of preschool-aged children in Kinshasa, Democratic Republic of the Congo. Pediatrics. 2008;122:e123–8. doi: 10.1542/peds.2007-2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Hare BA, Venables J, Nalubeg JF, Nakakeeto M, Kibirige M, Southall DP. Home-based care for orphaned children infected with HIV/AIDS in Uganda. AIDS Care. 2005;17:443–50. doi: 10.1080/09540120412331291779. [DOI] [PubMed] [Google Scholar]
- 20.Caldwell BM, Bradley RH. Home Observation for Measurement of the Environment. Little Rock, AR: University of Arkansas Press; 1979. [Google Scholar]
- 21.Bangirana P, John CC, Idro R, Opoka RO, Byarugaba J, Jurek AM, et al. Socioeconomic predictors of cognition in Ugandan children: Implications for community based interventions. PLoS ONE. 2009;4:e7898. doi: 10.1371/journal.pone.0007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mullen EM. Mullen Scales of Early Learning:AGS Edition. Minneapolis, MN: American Guidance Services; 1995. [Google Scholar]
- 23.Busman RA, Page CF, Oka E, Giordani B, Boivin MJ. Factors contributing to the psychosocial adjustment of Ugandan preschool children with HIV/AIDS. In: Boivin MJ, Giordani B, editors. Neuropsychology of Children in Africa: Perspectives on Risk and Resilience. New York: Springer; 2013. [Google Scholar]
- 24.Jordan CM, Johnson AL, Hughes SJ, Shapiro EG. The Color Object Association Test (COAT): the development of a new measure of declarative memory for 18- to 36-month-old toddlers. Child Neuropsychology. 2008;14:21–41. doi: 10.1080/09297040601100430. [DOI] [PubMed] [Google Scholar]
- 25.Achenbach TM, Rescorla LA. Manual for the ASEBA school-age forms & profiles: an integrated system of multi-informant assessment. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2001. [Google Scholar]
- 26.Achenbach TM, Rescorla LA. Multicultural supplement to the manual for the ASEBA school-age forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2007. [Google Scholar]
- 27.Bangirana P, Nakasujja N, Giordani B, Opoka RO, John CC, Boivin MJ. Reliability of the Luganda version of the Child Behaviour Checklist in measuring behavioural problems after cerebral malaria. Child Adolesc Psychiatry Ment Health. 2009;3:38. doi: 10.1186/1753-2000-3-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bass J, Neugebauer R, Clougherty KF, Verdeli H, Wickramaratne P, Ndogoni L, et al. Group interpersonal psychotherapy for depression in rural Uganda: 6-month outcomes: randomised controlled trial. Br J Psychiatry. 2006;188:567–73. doi: 10.1192/bjp.188.6.567. [DOI] [PubMed] [Google Scholar]
- 29.Boivin MJ, Bangirana P, Nakasuja N, Page CF, Shohet C, Givon D, et al. A year-long caregiver training program to improve neurocognition in preschool Ugandan HIV-exposed children. J Dev Behav Pediatr. 2013;34 doi: 10.1097/DBP.0b013e318285fba9. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Peterson NJ, Drotar D, Olness K, Guay L, Kiziri-Mayengo R. The relationship of maternal and child HIV infection to security of attachment among Ugandan infants. Child Psychiatry Hum Dev. 2001;32:3–17. doi: 10.1023/a:1017581412328. [DOI] [PubMed] [Google Scholar]
- 31.Caruso N. Refuge from the Lord’s Resistance Army in Uganda: a report from a Medecins Sans Frontieres team leader. Emerg Med Australas. 2006;18:295–8. doi: 10.1111/j.1742-6723.2006.00856.x. [DOI] [PubMed] [Google Scholar]
- 32.Fabiani M, Nattabi B, Opio AA, Musinguzi J, Biryahwaho B, Ayella EO, et al. A high prevalence of HIV-1 infection among pregnant women living in a rural district of north Uganda severely affected by civil strife. Trans R Soc Trop Med Hyg. 2006;100:586–93. doi: 10.1016/j.trstmh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 33.Orach CG, De Brouwere V. Integrating refugee and host health services in West Nile districts, Uganda. Health Policy Plan. 2006;21:53–64. doi: 10.1093/heapol/czj007. [DOI] [PubMed] [Google Scholar]
- 34.Foster G, Williamson J. A review of current literature on the impact of HIV/AIDS on children in sub-Saharan Africa. Aids. 2000;14 (Suppl 3):S275–84. [PubMed] [Google Scholar]
- 35.Boivin MJ, Green SD, Davies AG, Giordani B, Mokili JK, Cutting WA. A preliminary evaluation of the cognitive and motor effects of pediatric HIV infection in Zairian children. Health Psychol. 1995;14:13–21. doi: 10.1037//0278-6133.14.1.13. [DOI] [PubMed] [Google Scholar]
- 36.Abubakar A, Holding P, Van de Vijver FJ, Newton C, Van Baar A. Children at risk for developmental delay can be recognised by stunting, being underweight, ill health, little maternal schooling or high gravidity. J Child Psychol Psychiatry. 2009;51:652–9. doi: 10.1111/j.1469-7610.2009.02193.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Abubakar A, Holding P, Newton CR, van Baar A, van de Vijver FJ. The role of weight for age and disease stage in poor psychomotor outcome of HIV-infected children in Kilifi, Kenya. Dev Med Child Neurol. 2009;51:968–73. doi: 10.1111/j.1469-8749.2009.03333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abubakar A, Van Baar A, Van de Vijver FJ, Holding P, Newton CR. Paediatric HIV and neurodevelopment in sub-Saharan Africa: a systematic review. Trop Med Int Health. 2008;13:880–7. doi: 10.1111/j.1365-3156.2008.02079.x. [DOI] [PubMed] [Google Scholar]
- 39.Bonnier C. Evaluation of early stimulation programs for enhancing brain development. Acta Paediatr. 2008;97:853–8. doi: 10.1111/j.1651-2227.2008.00834.x. [DOI] [PubMed] [Google Scholar]
- 40.Farah MJ, Betancourt L, Shera DM, Savage JH, Giannetta JM, Brodsky NL, et al. Environmental stimulation, parental nurturance and cognitive development in humans. Dev Sci. 2008;11:793–801. doi: 10.1111/j.1467-7687.2008.00688.x. [DOI] [PubMed] [Google Scholar]
- 41.Rao H, Betancourt L, Giannetta JM, Brodsky NL, Korczykowski M, Avants BB, et al. Early parental care is important for hippocampal maturation: evidence from brain morphology in humans. Neuroimage. 2009;49:1144–50. doi: 10.1016/j.neuroimage.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vygotsky LS. Mind in Society: The Development of Higher Psychological Processes. Cambridge, MA: Harvard University Press; 1978. [Google Scholar]
- 43.Boivin MJ, Giordani B. Neuropsychological assessment of African children: Evidence for a universal basis to cognitive ability. In: Chiao JY, editor. Cultural Neuroscience: Cultural Influences on Brain Function. New York, NY: Elsevier Publications; 2009. pp. 113–35. [DOI] [PubMed] [Google Scholar]